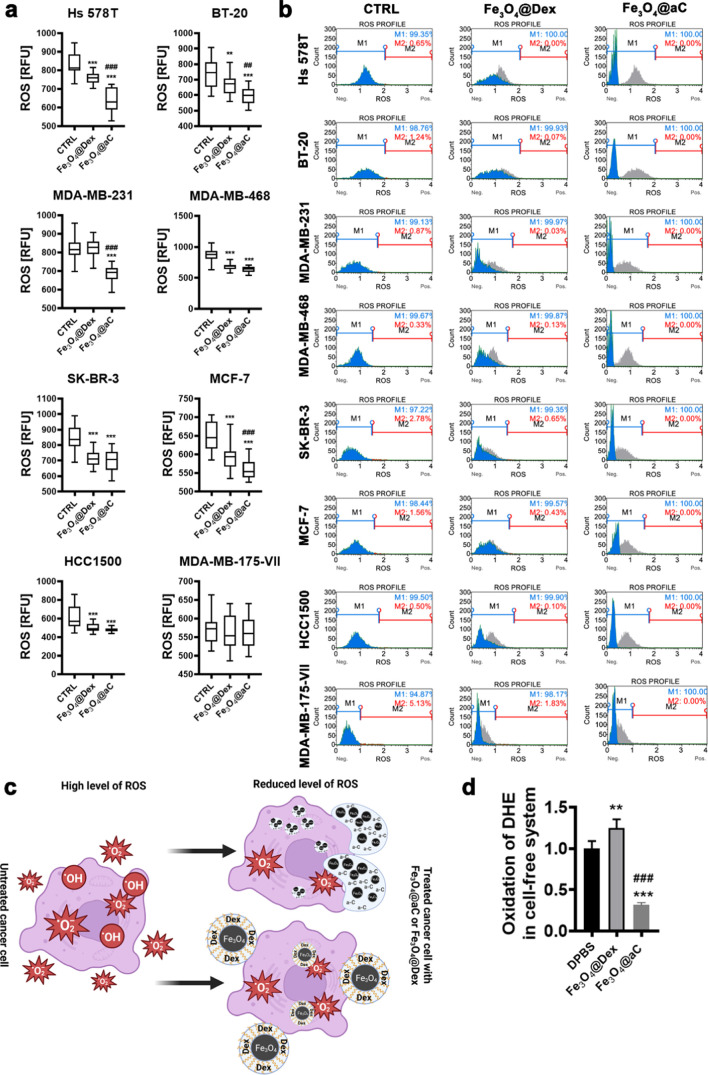
Figure 3.
Redox imbalance promoted by encapsulated Fe3O4 NPs in breast cancer cells (a–c) and redox activity of encapsulated Fe3O4 NPs in a cell-free in vitro system (d). Cells were treated with 100 μg/mL NPs for 4 h, and total ROS levels (a) and total superoxide levels were analyzed using dedicated fluorogenic probes and imaging and flow cytometry, respectively. (a) Total ROS levels are presented in relative fluorescence units (RFU). Box and whisker plots are shown, n = 3, ***p < 0.001, and **p < 0.01 compared to untreated control (ANOVA and Dunnett’s a posteriori test); ###p < 0.001 and ##p < 0.01 compared to Fe3O4@Dex treatment (ANOVA and Tukey’s a posteriori test). (b) Representative histograms are shown. Superoxide levels are denoted as ROS. Blue histogram (M1), superoxide-negative population; red histogram (M2), superoxide-positive population; and gray histogram, ROS profile at control untreated conditions. (c) Graph illustrating reductive stress induced by encapsulated Fe3O4 NPs in breast cancer cells. Created with BioRender.com. (d) Dihydroethidium-based fluorescence in DPBS in the presence and the absence of encapsulated Fe3O4 NPs. Data were normalized to control (relative fluorescence units, RFU of dihydroethidium in DPBS). Bars indicate SD, n = 3, ***p < 0.001, and **p < 0.01 compared to dihydroethidium in DPBS (ANOVA and Dunnett’s a posteriori test); ###p < 0.001 compared to Fe3O4@Dex action (ANOVA and Tukey’s a posteriori test). CTRL, untreated control; DHE, dihydroethidium; DPBS, Dulbecco’s phosphate-buffered saline; Fe3O4@Dex, dextran-based coated iron oxide nanoparticles; and Fe3O4@aC, glucosamine-based amorphous carbon-coated iron oxide nanoparticles.