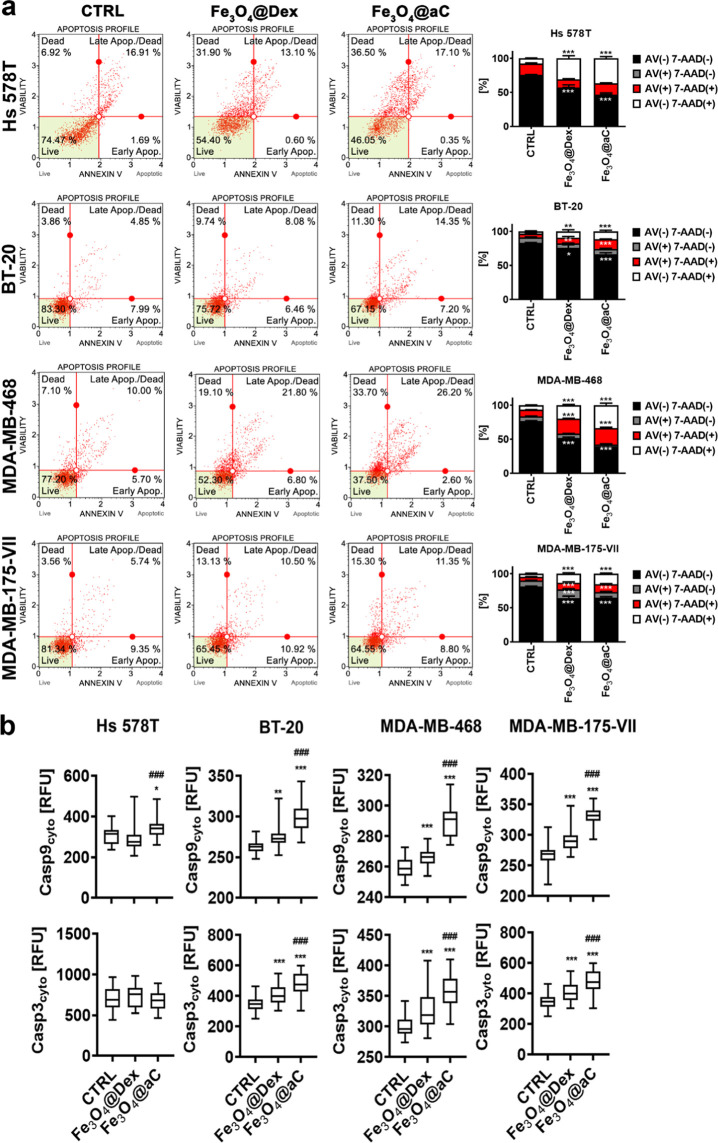
Figure 5.
Apoptosis and necrosis induced by encapsulated Fe3O4 NPs in drug-induced senescent breast cancer cells. The senescence program was activated using etoposide treatment. Senescent breast cancer cells were treated with 100 μg/mL NPs for 4 h, and apoptotic and necrotic cell death was revealed using Annexin V (phosphatidylserine externalization, an apoptotic marker) and 7-AAD (rupture of plasma membrane, a necrotic marker) dual staining and flow cytometry (a). Representative dot plots are shown. (b) Bars indicate SD, n = 3, ***p < 0.001, **p < 0.01, and *p < 0.05 compared to untreated control (ANOVA and Dunnett’s a posteriori test). Four subpopulations are shown, namely, live cells (Annexin V (AV)-negative, 7-AAD-negative), early apoptotic cells (Annexin V (AV)-positive, 7-AAD-negative), late apoptotic cells (Annexin V (AV)-positive, 7-AAD-positive), and necrotic cells (Annexin V (AV)-negative, 7-AAD-positive). (b) Apoptosis was also studied using the analysis of the levels of caspase 9 and caspase 3. The levels of caspase 9 and caspase 3 were assayed using immunostaining and imaging cytometry. The levels of caspase 9 and caspase 3 are presented as relative fluorescence units (RFU). Box and whisker plots are shown, n = 3, ***p < 0.001, **p < 0.01, and *p < 0.05 compared to untreated control (ANOVA and Dunnett’s a posteriori test); ###p < 0.001 compared to Fe3O4@Dex treatment (ANOVA and Tukey’s a posteriori test). CTRL, untreated control; Fe3O4@Dex, dextran-based coated iron oxide nanoparticles; and Fe3O4@aC, glucosamine-based amorphous carbon-coated iron oxide nanoparticles.