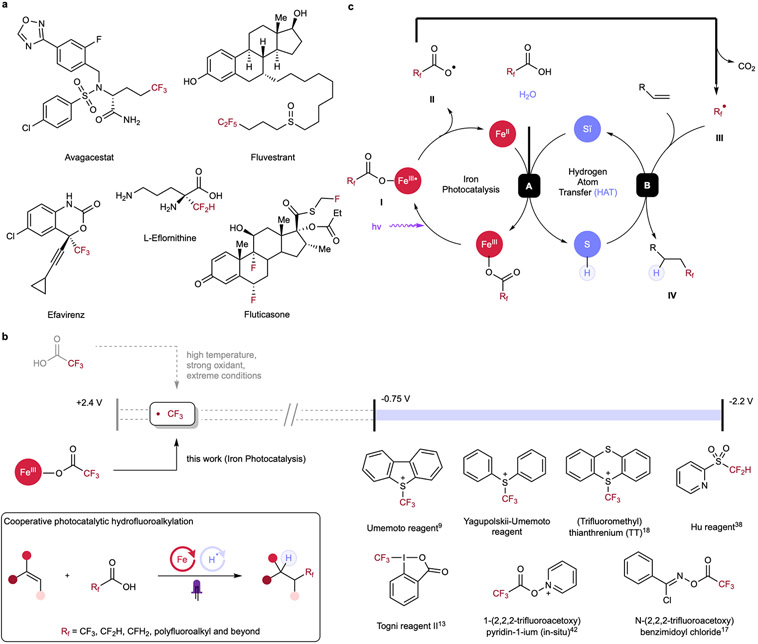
Figure 1. Hydrofluoroalkylation of alkenes for accessing valuable fluorinated molecules.
(a) Selected examples of fluorine-containing pharmaceuticals. (b) Fluoroalkyl radicals are challenging to generate directly from fluoroalkyl carboxylic acids due to their high oxidation potential, necessitating the use of complex and expensive precursor reagents. Cooperative photocatalytic hydrofluoroalkylation allows simple, mild, and broadly-applicable syntheses of valuable fluorinated molecules by direct activation of fluoroalkyl carboxylic acids, addressing limitations including the use of noble metals, electrophilic/expensive agents and harsh conditions in previous methods. (c) Postulated mechanism of the photocatalytic hydrofluoroalkylation. Upon light irradiation, homolytic cleavage of fluoroalkyl carboxylate is induced, forming carboxyl radical II and reduced FeII. Radical II undergoes CO2 extrusion and generates fluoroalkyl radical III, which can then engage in radical addition to the alkene and forming an alkyl radical. The thiol co-catalyst can then sequester this transient alkyl radical intermediate via hydrogen atom transfer (HAT) to form hydrofluoroalkylated product and a thiyl radical. Finally, redox interaction between reduced FeII and thiyl radical in the presence of a proton source allows both catalytic cycles to be closed, enabling cooperative photocatalytic hydrofluoroalkylation.