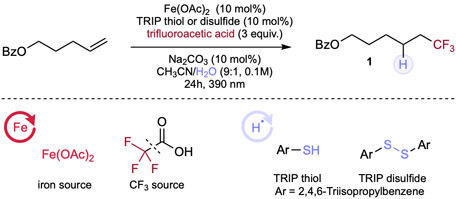
Table 1.
Optimization of alkene hydrotrifluoromethylation.
| ||
|---|---|---|
| Entry | Deviation from Standard Conditions | Yield (%)a |
| 1 | none | 86(84)b, 92(89)c |
| 2b | [Cu(MeCN)4]BF4, Cu(OTf)2 | ND |
| 3d | Fe(acac)3, FeCl3·6H2O, Fe(NO3)3·9H2O, Fe(OTf)2, FeCl2 | trace–72 |
| 4 | 2 equiv. of TFA | 74 |
| 5b | no base; 100 mol% of Na2CO3 | 72; 62 |
| 6b | DCM, THF | trace–20 |
| 7b | Acetone, EA | 42–76 |
| 8 | no iron salt | ND |
| 9 | no light | ND |
| 10b | no HAT reagent | trace |
| 11 | no water | 18b, 24c |
Reaction conditions: alkene (0.1mmol, 1.0 equiv.), trifluoroacetic acid (3.0 equiv.), Fe(OAc)2 (10 mol%), TRIP thiol or disulfide (10 mol%), Na2CO3 (10 mol%) and solvent (0.1 M), 24h, RT, 390nm Kessil blue LED. a 1H NMR yield is determined by using CH2Br2 as an internal standard. Isolated yield in the parentheses. b With 10 mol% of TRIP thiol. c With 10 mol% of TRIP disulfide. d With 5 mol% of iron salt and 5 mol% TRIP disulfide.
