Table 3.
Hydrotrifluoromethylation and hydrodifluoromethylation of APIs & natural product alkene derivatives.
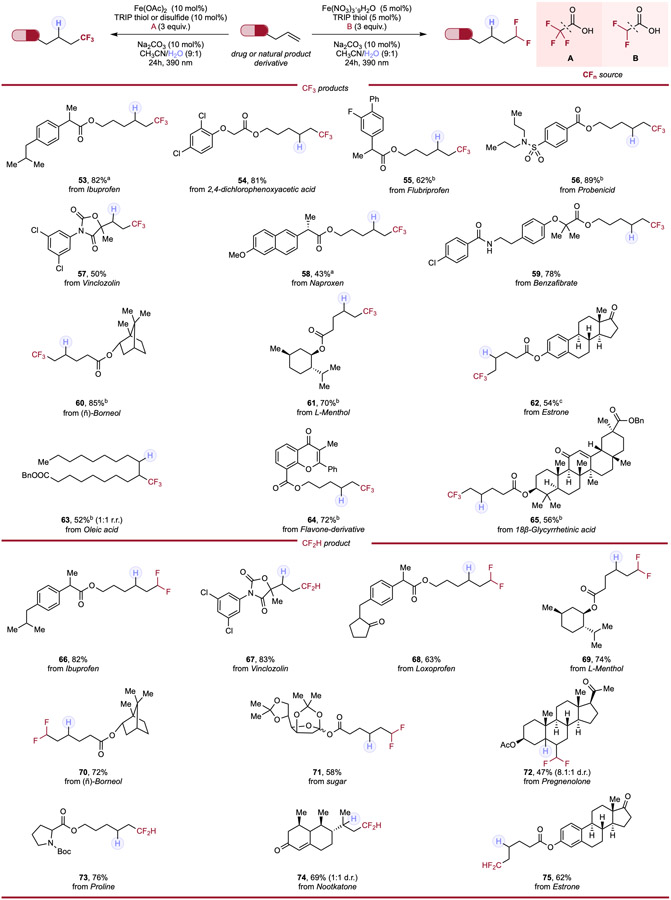
|
Reaction conditions for hydrotrifluoromethylation: alkene (0.1mmol, 1.0 equiv.), trifluoroacetic acid (3.0 equiv.), Fe(OAc)2 (10 mol%), HAT reagent (10 mol%), Na2CO3 (10 mol%) and CH3CN/H2O (9:1, 0.1 M), 24h, RT, 390nm Kessil blue LED. Reaction conditions for hydrodifluoromethylation: alkene (0.1mmol, 1.0 equiv.), difluoroacetic acid (3.0 equiv.), Fe(NO3)3·9H2O (5 mol%), TRIP thiol (5 mol%), Na2CO3 (10 mol%) and CH3CN/H2O (9:1, 0.1 M), 24h, RT, 390nm Kessil blue LED. a With 10 mol% of TRIP thiol. b With 10 mol% of TRIP disulfide.c With Fe(OAc)2 (20 mol%), TRIP thiol (20 mol%) and trifluoroacetic acid (6 equiv.).
