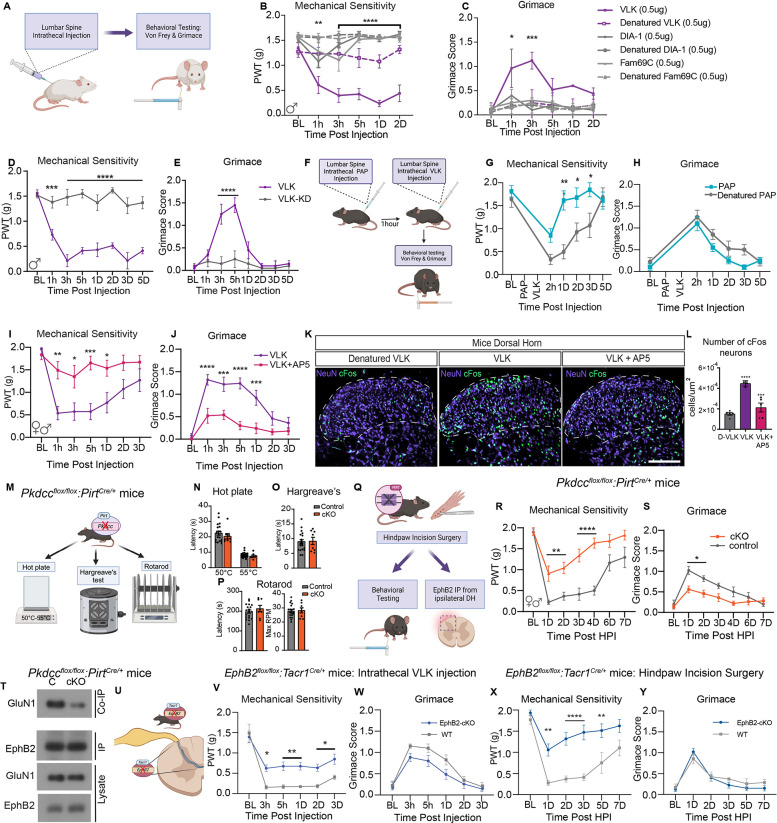
Fig. 4. VLK induces a robust pain state dependent on NMDA receptor activation, and endogenous expression in DRG sensory neurons is necessary for post-operative pain.
(A) Schematic illustration of intrathecal drug administration and measurement of pain-like behaviors. von-Frey testing is used as a measure of mechanical sensitivity and grimace scores depict levels of affective pain. (B, C) Three different secreted kinases and their denatured controls were administered to mice with 0.5μg of VLK, DIA-1 and FAM69C. Mice receiving VLK displayed a sharp drop in mechanical thresholds and increased grimacing. (n = 4, Two-way repeated measures ANOVA, F (5, 162) = 82. 33,**p=0.001, ****p<0.0001; F (5, 162) = 9.759, ***p<0.0001. post hoc Dunnett’s test). (D, E) Administration of a kinase-dead mutant of VLK (VLK-KD) abolishes pain-like behaviors induced by VLK. (n=5, Repeated measures ANOVA, F (2, 11) = 26. 19, ****p<0.0001; Two-way repeated measures ANOVA, F (2, 11) = 11.07, **p=0.0023. post hoc: Dunnett’s test). (F) Schematic of administration of the extracellular phosphatase PAP followed by VLK and measurement of pain-like behaviors. (G, H) Mice administered PAP followed by VLK showed significantly reduced mechanical sensitivity (n=8, Two-way repeated measures ANOVA, F (5, 70) = 18.96,***p=0.0003 Posthoc: Bonferroni) whereas PAP did not affect VLK-induced grimacing compared to denatured PAP control (n=8, Two-way repeated measures ANOVA, posthoc: Bonferroni) . (I, J) Co-administration of VLK with AP5 also blocked the VLK-induced pain phenotype. (n=8, Two-way repeated measures ANOVA F (1, 18) = 18.85, ***p=0.0004, F (1, 18) = 19.93, ****p=0.0003. post hoc: Bonferroni). (K) The lumbar spinal cord dorsal horn from mice administered Denatured VLK, VLK, or VLK + AP5 was immunohistochemically stained for NeuN and cFos markers. (L) Quantification of cFos+ cells showed VLK induced a significant increase in neuronal cFos expression which was blocked by AP5 antagonism of NMDARs or with the use of heat Denatured VLK (D-VLK). (One-way ANOVA with Bonferroni multiple comparisons ****p<0.0001, ***p<0.001 (M) Model depicting Pkdccflox/flox PirtCre/+ mice (cKO) and Pkdccflox/flox littermate controls (Control) were subjected to behavioral testing for normal sensorimotor development. (N-P) Paw withdrawal latencies for were measured on a Hot Plate in (N) and Hargreaves assay in (O). Rotarod testing was performed in (P). No significant differences were observed between genotypes in the above tests. (Q) A model of the Pkdcc conditional knockout mice (cKO). Pkdcc-flox mice were crossed to Pirt-Cre line to generate Pkdcc sensory-neuron specific cKO mice. cKO and wild-type littermates were subjected to Hind-paw incision surgeries. (R-S) Behavioral data showing effects of sensory neuron-specific VLK knockout on postsurgical mechanical hypersensitivity and affective pain. (n = 9 CKO, n = 8 Littermate; Two-way repeated measures ANOVA F (1, 16) = 21. 38, ***p=0.0003; F (1, 16) = 9.902, **p=0.0062. posthoc: Bonferroni). (T) Immunoblot showing levels of GluN1 co-immunoprecipitated with EphB2 in the ipsilateral dorsal horn (DH) in cKO mice compared to wildtype littermate controls (C). A fraction of the same sample was immunoblotted for EphB2 (EphB2, IP). DH lysates were probed as indicated for EphB2 and GluN1. (U) Illustration depicting intrathecal injection of recombinant VLK in EphB2flox/flox Tacr1Cre/+ mice (EphB2 cKO). (V-W) Measurement of pain-like behaviors using Von Frey (V) (n=7, Two-way repeated measures ANOVA, F (5, 65) = 2.641, **p=0.003, posthoc: Bonferroni) and Grimace (W) (n=7, p=NS, posthoc: Bonferroni). (X-Y) Behavioral data (Von-Frey and Grimace) of EphB2flox/flox Tacr1Cre/+ mice (EphB2 cKO) following Hindpaw Incision surgery. Mechanical Sensitivity measured using Von Frey (X) (n = 8 CKO, n = 7 Littermate; Two-way repeated measures ANOVA F (5, 65) = 3.557, **p=0.0066, ****p<0.0001, posthoc: Bonferroni), Grimace (Y) (n = 8 CKO, n = 7 Littermate; Two-way repeated measures ANOVA p=ns, posthoc: Bonferroni).