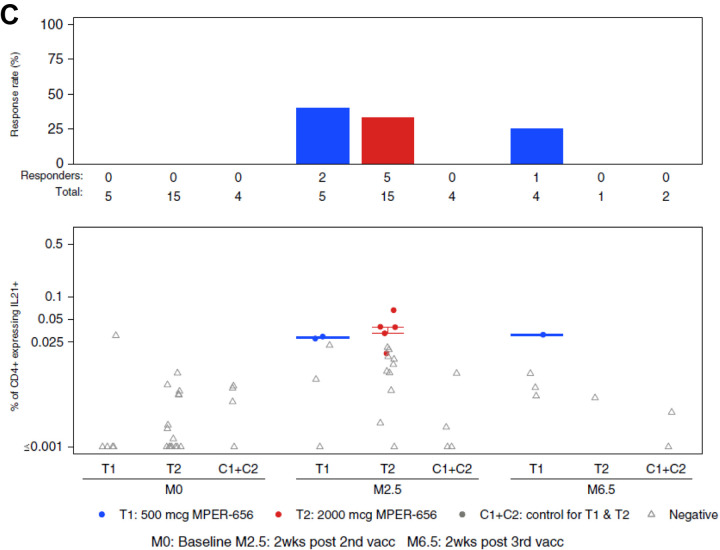
Figure 5. Intracellular Cytokine Staining (ICS) Results.
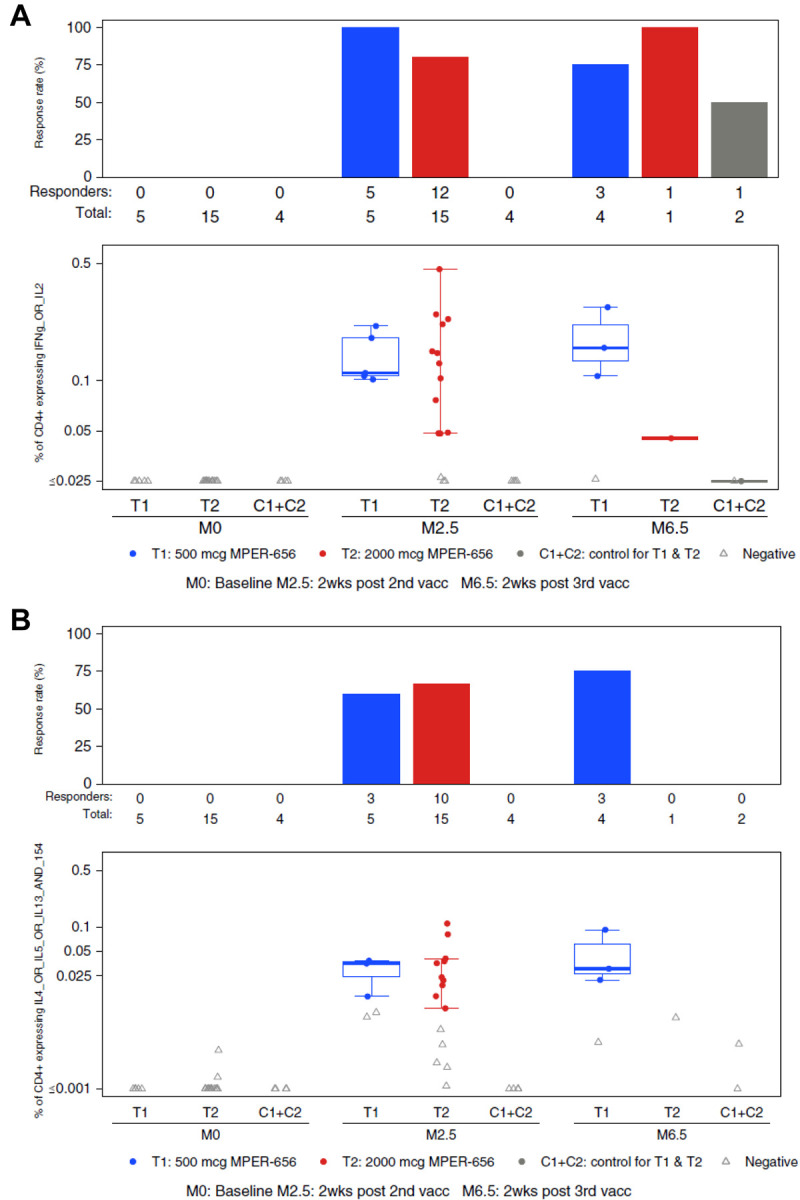
Endpoints reported were the response rates and the magnitudes of T-cell responses as measured by the ICS assay from PBMC specimens obtained at visits 2 (Month 0; baseline), 5 (Month 2.5; 2 weeks post 2nd vaccination), and 7 (Month 6.5; 2 weeks post 3rd vaccination). Vaccine trial participants included recipients of low (group T1) and high (group T2) dose vaccines as well as placebo (C1+C2). (A) HVTN 133 ICS CD4+ T cell IFNg_OR_IL2 expression in response to HIV-gp41 MPER. (B) HVTN 133 ICS CD4+ T cell IL4_OR_IL5_OR_IL13_AND_154 Expression in Response to HIV-gp41 MPER. (C) HVTN 133 ICS CD4+ T cell IL21+ Expression in Response to HIV-gp41 MPER. PBMCs collected from participants were stimulated by gp41 peptide pools and were assayed for the presence of Th1-type cytokines (IL-2, IFNy; A), Th2-type cytokines (IL-4, IL-5, IL-13, CD40L; B), and IL-21 (C). There was one positive, but very low magnitude IFN-γ and/or IL-2 CD4+ T-cell response to HIV-gp41 MPER in the pooled placebo groups (C1+C2).