FIGURE 3.
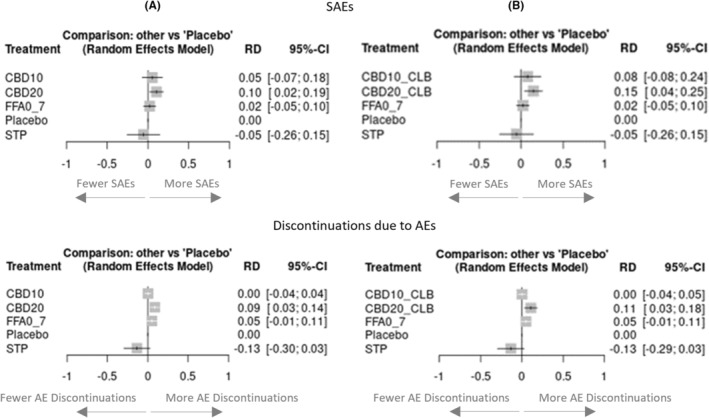
Pairwise risk differences versus placebo for SAEs and Discontinuations due to AEs. (A) Risk differences using full cannabidiol trial populations; (B) Risk differences using subgroup of cannabidiol trial populations taking clobazam. AEs, adverse events; CBD10, cannabidiol 10 mg/kg/day; CBD10_CLB, cannabidiol 10 mg/kg/day + clobazam; CBD20, cannabidiol 20 mg/kg/day; CBD20_CLB, cannabidiol 20 mg/kg/day + clobazam; FFA0_7, fenfluramine 0.7 mg/kg/day; RD, risk difference; SAEs, serious adverse events; STP, stiripentol 50 mg/kg/day; 95% CI, 95% confidence intervals.
