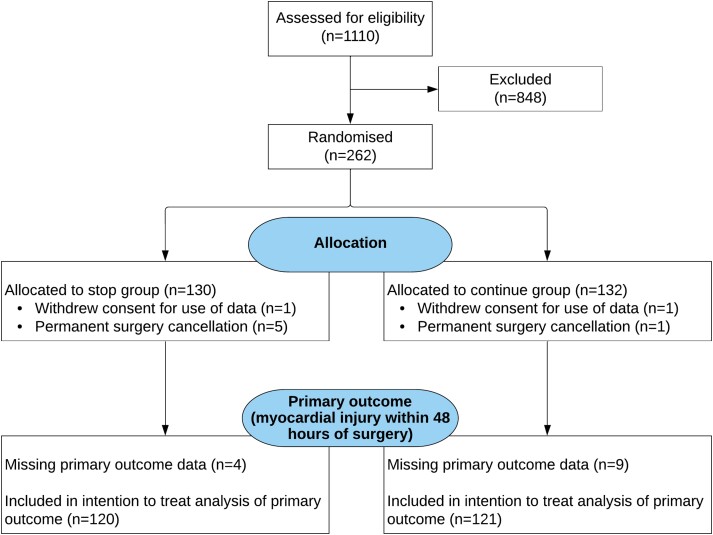
Figure 1.
CONSORT diagram. Details are shown regarding the screening, potential eligibility, randomized assignments, and disposition of the trial patients. Details regarding the methods that were used for screening and the determining of eligibility are provided in the Supplementary data online, Appendix. Before surgery, there were 6 patients for whom surgery was cancelled after randomization had occurred (for surgical/oncologic reasons). Two additional patients withdrew their consent after randomization but before attending for surgery. Once admitted for surgery, primary outcome data was missing for 4 patients allocated to stop their RAS inhibitor, and in 9 patients randomized to continue their RAS therapy. The primary outcome could not be met if the pre-operative sample was not collected or the day 1 and day 2 samples were not collected even though the pre-operative sample had been. The reasons for this were either that 11 patients refused to give all the required additional blood sample (but consented to continue in the trial) or samples were not obtained by research staff (n = 2).