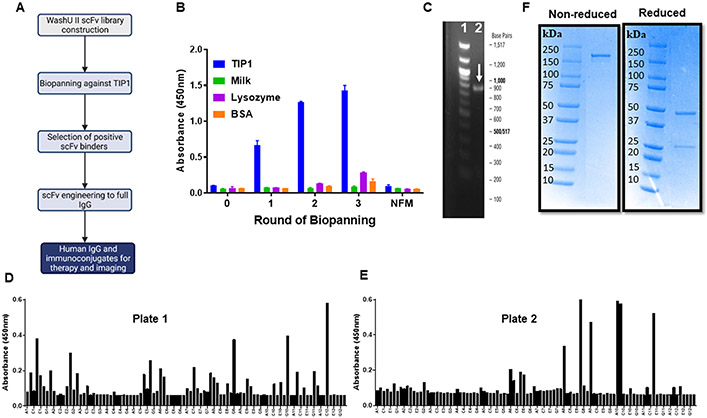
Figure 1. Anti-TIP1 human scFv discovery and engineering to a full-length IgG.
A. Schematic representation of the steps for antibody discovery and engineering using the WashU II phage-display library. B. Bar graph of polyclonal phage ELISA showing enrichment of TIP1 specific scFvs with three rounds of biopanning. The ELISA plates were coated with TIP1, Milk, lysozyme, or BSA and probed with phages eluted from three rounds of biopanning with the recombinant TIP1 protein. NFM: Non-fat milk. C. Representative DNA gel showing the amplification of a full-length scFv from the phagemid. Lane 1 represents the 100 bp DNA ladder with the indicated base pairs outside the gel image. Lane 2 is the amplified scFv indicated with a white arrow. D and E. Two different 96-well plates of monoclonal phage ELISA showing positive anti-TIP1 scFv binders. The ELISA plates were coated with recombinant TIP1 protein and probed with monoclonal phages obtained after the three rounds of biopanning. Each bar represents a single phage clone. F. Purified human L111 antibody was resolved on SDS-PAGE under non-reducing and reducing conditions. L111 is observed above 150 kDa under non-reducing conditions. Heavy and light chains are observed in the reducing condition at 50 kDa and 25 kDa, respectively.