Figure 6:
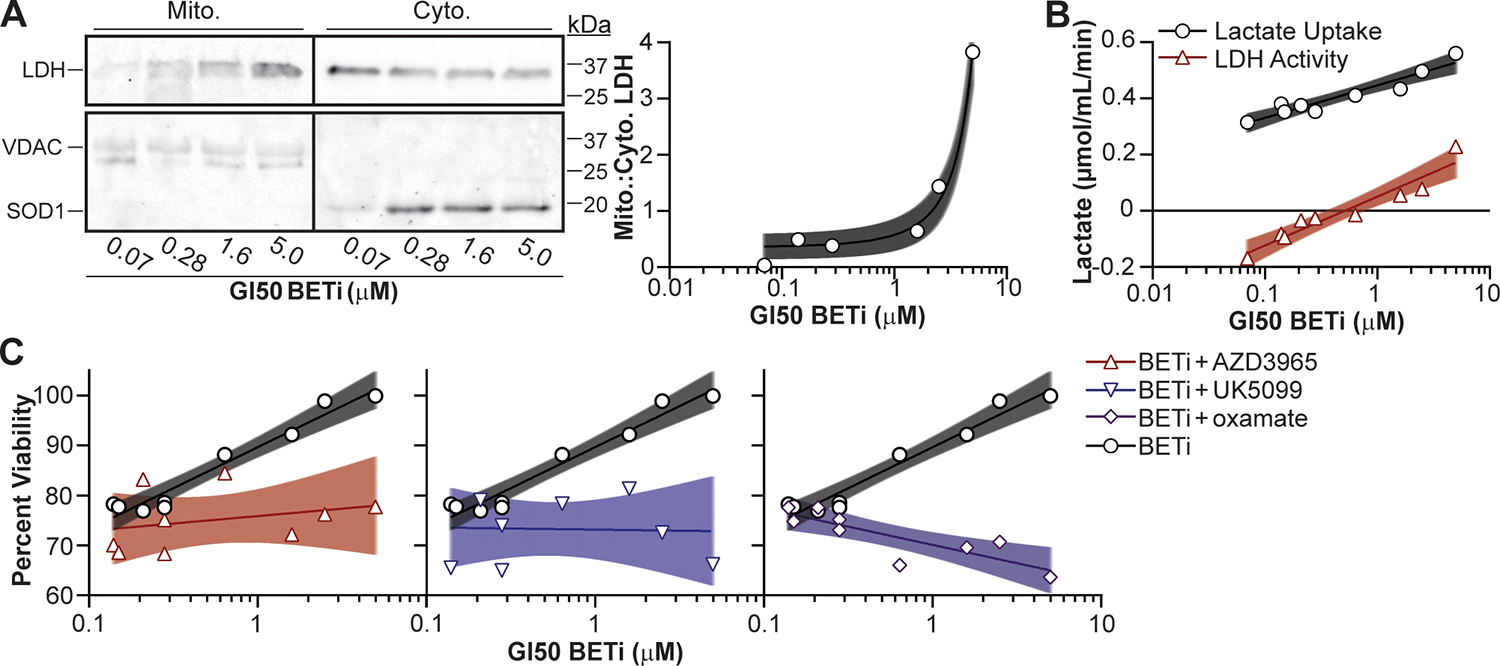
Lactate utilization facilitates BETi resistance in AML patient myeloblasts. (A) Patient myeloblasts with defined GI50 BETi concentrations were lysed, fractionated, and LDH protein in the cytosolic (Cyto.) and mitochondrial (Mito.) fractions quantified by immunoblot. SOD1 and VDAC protein were immunoblotted to ensure clean fractionation. (B) Cells were treated with BETi, and the media exchanged after 48 hr with minimal media with and without oxamate (25 μM) or lysed to quantify baseline metabolite levels. Minimal media was subsequently supplemented with lactate (20 μM) for 2 hr, cells lysed, and intracellular lactate levels quantified using a fluorescent plate reader (Lactate Uptake = oxamate-treated – baseline; LDH Activity = oxamate-treated – mock-treated). (C) Myeloblasts were cultured for 72 hr with BETi (0.15 μM) and/or lactate utilization inhibitors (AZD3965, UK5099, oxamate = 0.1 μM) and viability quantified using a fluorescent plate reader. Viability was quantified relative to vehicle control treated cells. Each point represents the mean (technical duplicate) value obtained from an individual patient. Shaded regions represent 95% confidence bands.
