Abstract
Cancer immunotherapy has garnered promise in tumor progression, invasion, and metastasis through establishing durable and memorable immunological activity. However, low response rates, adverse side effects, and high costs compromise the additional benefits for patients treated with current chemical and biological agents. Chinese herbal medicines (CHMs) are a potential treasure trove of natural medicines and are gaining momentum in cancer immunomodulation with multi-component, multi-target, and multi-pathway characteristics. The active ingredient extracted from CHMs benefit generalized patients through modulating immune response mechanisms. Additionally, the introduction of nanotechnology has greatly improved the pharmacological qualities of active ingredients through increasing the hydrophilicity, stability, permeability, and targeting characteristics, further enhancing anti-cancer immunity. In this review, we summarize the mechanism of active ingredients for cancer immunomodulation, highlight nano-formulated deliveries of active ingredients for cancer immunotherapy, and provide insights into the future applications in the emerging field of nano-formulated active ingredients of CHMs.
KEY WORDS: Chinese herbal medicines, Nano-formulation, Active ingredients, Drug delivery, Cancer immunotherapy, Innate immune system, Adaptive immune system
Graphical abstract
Nano-formulated active ingredients from Chinese herbal medicines demonstrate cancer immunomodulation through interacting with the innate and adaptive immune systems and modulating the microenvironment signal molecules.

1. Introduction
Cancer immunotherapy activates the host's own immune system to induce long-term immunity through defending against tumor invasion, inhibiting tumor progression, and relieving immune tolerance1. The innate and adaptive immune system determines the specificity of the anti-tumor response, which is associated with corresponding immune cells and regulatory signal molecules. Innate immune cells like macrophage, dendritic cells (DCs) and natural killer cells (NKs) serve as a natural protective barrier to hinder tumor invasion and eradicate tumor cells, while mediating the initiation of the antigen presentation process for adaptive immune responses2, 3, 4. In response to tumor-specific antigens, adaptive immune cells (i.e., B and T cells) are activated to induce long-term, specific, and memorable immunity against tumor progression. Additionally, the immunosuppressive tumor microenvironment contains pro-tumor cytokines and immune checkpoint molecules that restrict the systemic immune response resulting in immune resistance5, 6, 7. Therefore, activating the immune system and tailoring the microenvironment signal molecules to restore immune surveillance are essential to cancer immunotherapy with demonstrated clinical translations. Recently, immune checkpoint antibodies of Ipilimumab, Pembrolizumab and the cellular therapeutic Kymriah are approved for clinical treatment8. However, the high costs, off-target effects, and adverse side effects compromised the benefits of patients9, thus facilitating the development of novel therapeutic strategies.
Chinese herbal medicines (CHMs) originate from natural materials and are considered as a prospective cancer treatment strategy10, 11, 12. Compared with chemical and biological agents, active ingredients extracted from CHMs with mild and broad-spectrum efficacy avoid potential challenges of high costs, adverse side effects, and multidrug resistance associated with chemotherapeutics, contributing to an improved quality of life and extended patient survival. Active ingredients of CHMs possess superior immunomodulation abilities from multi-channel, multi-target, and multi-level characteristics that have demonstrated tremendous immunotherapeutic potential for cancer immunotherapy and exerting anti-cancer effects13,14. These modulate the innate immune response, adaptive immune response and the complicated microenvironment signal to induce long-term and memorable systemic immune response against tumor progression, invasion, and metastasis15, 16, 17. However, adverse pharmacological properties neutralize its therapeutic efficacy such as hydrophobicity, low stability, inadequate permeability, and a short half-life18, 19, 20. Leveraging carrier material to maximize the bioavailability and bioactivity of active ingredients of CHMs is a synergizing strategy for cancer immunotherapy.
The emergence of nanotechnology, specifically the integration of nanomaterials and drugs, has revolutionized disease diagnosis and treatment21,22. Nanomaterials such as polymers, liposomes, inorganic materials, and nanofibers have attracted widespread attention on clinical translation, facilitating the achievement of precise medicine treatment. By engineering nano-formulated active ingredients of CHMs, the bioavailability and bioactivity of active pharmaceutical agents can be improved through increasing its solubility, stability, permeability, and half-life characteristics23. This mode of pharmaceutical transport can also target cancer tissues and cells more effectively, avoiding poor response rates and reducing blood circulation times. Additionally, nano-formulated active ingredients of CHMs are capable of on-demand release in a controlled and sustained manner, collectively minimizing undesirable adverse events and maximizing cancer immunity24.
In this review, we summarize the modulatory mechanisms of active ingredients of CHMs for cancer immunotherapy through activating the innate immune response, adaptive immune response, and controlling the complicated tumor microenvironment signal (Fig. 1). By introduction of nanomaterials (i.e., polymers, liposomes, inorganic substances, extracellular vesicles, and nanofibers), nano-formulated active ingredients of CHMs contribute to improving the bioavailability and bioactivity. Additionally, the prospects and challenges of nano-formulated active ingredients from CHMs related to process feasibility, industrial production, and safety concerns are discussed in this review.
Figure 1.
Nano-formulated active ingredients from CHMs demonstrate cancer immunomodulation through interacting with the innate and adaptive immune systems, and modulating the microenvironment signal molecules.
2. The mechanism of active ingredients from CHMs for cancer immunotherapy
Cancer immunotherapy is a promising strategy to extend patient survival by reeducating the host's immune system and has been used in clinical translation such as CpG 1018, the biological drug in Nivolumab, Kymriah25,26. However, low response ratios, severe side effects, and high costs negatively impact the efficacy and availability of immunotherapeutic to patients. CHMs serve as a potential solution to expand patient-centered benefits as they are naturally derived pharmaceutical resources with multi-targeting abilities for disease prevention and treatment27. Active ingredients found in CHMs in (Table 1)3,4,6,7,11,12,16,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 such as polysaccharides, flavonoids, saponins, and alkaloids have demonstrated robust immunological activity against tumor cells44,62,66. Additionally, CHMs with multi-channel immunomodulatory characteristics can utilize their powerful active ingredients in multiple pathways to eliminate tumor cells, such as modulating the innate and adaptive immune systems, and influencing microenvironment signal molecules.
Table 1.
Active ingredients of CHMs and corresponding immunomodulatory mechanisms.
| Active ingredient | CHM | Mechanism | Ref. |
|---|---|---|---|
| Artesunate | Artemisia carvifolia Buch. Ham. ex Roxb. Hort. Beng. | Tregs reduction TGF-β1, IL-10 reduction |
6,28 |
| Angelica polysaccharide | Angelica sinensis (Oliv.) Diels | NKs activation Th1/Th2 regulation |
29,30 |
| Astragalus polysaccharide | Astragalus membranaceus | Macrophage polarization DCs maturation PD-L1 reduction |
7,31 |
| Bufalin | Bufo bufo gargarizans Cantor | Macrophage polarization NKs activation |
32,33 |
| Berberine | Coptis chinensis Franch. | CTLs activation PD-L1 reduction |
34 |
| Curcumin | Curcuma longa L. | Tregs reduction Th1/Th2 regulation |
12,35 |
| Cryptotanshinone | Salvia miltiorrhiza Bunge | DCs maturation | 36 |
| Dioscin | Discorea nipponica Makino | Macrophage polarization | 37 |
| Dihydroartemisinin | Artemisia annua L. | Macrophage polarization | 11 |
| Epigallocatechin gallate | Camellia sinensis | Macrophage polarization PD-L1 reduction |
38,39 |
| Epimedium polysaccharide | Epimedium pseudowushanense | DCs maturation | 3 |
| Ginsenoside Rh2 | Panax ginseng C. A. Mey | Macrophage polarization | 40,41 |
| Ginsenoside Rg3 | Panax ginseng C. A. Mey | PD-L1 reduction | 16 |
| Ginseng polysaccharides | Panax ginseng C. A. Mey. | NKs activation Tregs reduction |
42,43 |
| Ganoderma formosanum polysaccharides | Ganoderma sinense | DCs maturation | 44 |
| Icariin | Epimedium brevicornu Maxim. | M2 reduction | 45 |
| Luteolin | Reseda odorata L. | Tregs reduction | 46 |
| Lentinan | Lentinus edodes | CTLs activation Tregs, MDSCs reduction |
47,48 |
| Lycium barbarum polysaccharides | Lycium barbarum L. | Macrophage polarization DCs maturation CTLs activation |
49,50 |
| Norcantharidin | Mylabris phalerata Pallas | Macrophage polarization Tregs reduction |
51,52 |
| Oridonin | Rabdosia rubescens (Hemsl.) Hara | Th1/Th2 regulation | 53 |
| Resveratrol | Veratrum album L. | Tregs reduction PD-1 reduction Macrophage polarization |
54,55 |
| Rehmannia glutinosa | Rehmannia glutinosa (Gaert.) Libosch. ex Fisch. et M | DCs maturation NKs activation |
56,57 |
| Sativan | Spatholobus suberectus Dunn | PD-L1 reduction | 58 |
| Shikonin | Lithospermum erythrorhizon Sieb. et Zucc. | DCs maturation NKs proliferation |
59,60 |
| Solamargine | Solanum nigrum L. | Macrophage polarization | 61 |
| SaikosaponinA | Bupleurum chinense DC. | Th1/Th2 regulation | 62 |
| Triptolide | Tripterygium wilfordii Hook. f. | Tregs reduction PD-L1 reduction |
63,64 |
| Tetramethypyrazine | Ligusticum chuanxiong Hort | NKs activation | 4 |
| β-Elemene | Curcuma wenyujin Y.H. Chen & C. Ling | Macrophage polarization | 65 |
2.1. Modulate the innate immune system
The innate immune system, a first line response to pathogenic invasion, acts as a non-specific and non-memorable protective barrier for defending against infection. Innate immune cells such as macrophages, DCs, and NKs rapidly respond to invading pathogens and further produce non-specific anti-infective immunity44,45,56,67. Innate immunity also establishes a foundation and interacts with adaptive immunity through macrophages and DCs presenting pathogen-associated antigens to T cells in acquired immune cells to activate adaptive immune responses46,48,53.
2.1.1. Modulate the macrophages
Macrophages are highly plastic cells that contain two subtypes, the M1 (anti-tumor) and M2 (pro-tumor), which are driven by surrounding cytokines and the tissular niche37,65,68. M1 macrophages predominantly mediate tumor cell death by cytotoxicity, phagocytosis, or vascular damage. Conversely, M2 macrophages, serve as collaborators in tumor progression and immune evasion by secreting anti-inflammatory cytokines, like transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), interleukin-4 (IL-4), and interleukin-10 (IL-10)51,52,69. Cancer progression and prognosis are highly associated with the ratio of M1 and M2 macrophages because the predominated M1 macrophage could polarize towards the M2 macrophage with uncontrolled tumor proliferation40,41. Therefore, reversing macrophage polarization or increasing the proportion of M1 macrophage is an effective immunological approach against malignancies32,33,70. The polyporus polysaccharide, extracted from traditional Chinese medicine Polyporus umbellatus, serves as an active ingredient with immunomodulatory, anti-tumor, anti-inflammatory, and hepatoprotective activities38,39,49. Liu et al.71 demonstrated that polyporus polysaccharide promoted the secretion of pro-inflammatory factors interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and inducible nitric oxide synthase (iNOS) and increased macrophage markers CD16, CD23, CD86, and CD40, possibly through activating NF-κB/NLRP3 pathways and upregulating the expression of NLRP3, p-P65, p-IκB and p-IKK-α/β in the tumor microenvironment to promote macrophage polarization. This polarization of macrophages in the tumor microenvironment towards the M1-like phenotype effectively inhibited the growth and migration of bladder cancer. In a similar immunomodulatory mechanism, solamargine, an active ingredient of Solanum nigrum L. repolarized M2 macrophages to an M1-like phenotype to alleviate the tumor microenvironment in a hepatocellular carcinoma model61.
2.1.2. Modulate the dendritic cells
DCs are the body's most potent, specialized antigen-presenting cells—capable of efficiently ingesting, processing and presenting antigens to link innate and adaptive immune responses. Immature DCs have an excellent capacity for migration and antigen phagocytosis; and these cells can differentiate into mature DCs after being triggered by antigen uptake or an external stimulus56. Mature DCs play a pivotal role in initiating the immune response program through activating and sustaining adaptive immune cells and inducing long-term immunological memory; and contain high amounts of major histocompatibility complex and co-stimulatory molecules that stimulate DCs and native T lymphocyte interactions36,44,72. Recently, DCs-based vaccines are moving to the forefront of cancer immunotherapy with sipuleucel-T (Provenge) being the first authorized oncologic autologous DCs vaccine for prostate cancer73. To enhance the efficacy of DCs-based vaccines, various medicinal plants have been used as immunomodulators for the maturation of DCs. As a potential adjuvant for a DCs-based breast cancer vaccine, polysaccharides purified from Portulaca oleracea L. have been utilized to promote the activation and maturation of DCs through binding toll-like receptor 4 (TLR4) and further activating nuclear transcription factor kappaB (NF-κB) pathway. The polysaccharide-assisted DCs vaccine reduced the tumor burdens and lung metastases, which might be attributed to accelerating DCs maturation, and improving antigen presentation by altering the TLR4/MyD88/NF-κB pathway by polysaccharides74. The CHMs of Lithospermum erythrorhizon and Salvia miltiorrhiza Bunge demonstrate immunomodulatory activity on DCs, where its active ingredients of shikonin and cryptotanshinone effectively enhanced immunogenicity and elicited anti-proliferative properties against lung cancer and breast cancer, respectively36,59,60.
2.1.3. Modulate the natural killer cells
NKs are innate lymphocytes capable of recognizing and directly killing cancer cells by releasing granzyme B, perforin, and cytokines via the antibody-dependent cell-mediated cytotoxicity effect75,76. NKs recognize their targets in a human leukocyte antigen-independent manner through germline-encoded receptors that recognize and bind to cell surface ligands, offering a unique advantage for cancer immunotherapy in which cancer cells can be eliminated without risking T cell-driven inflammatory cytokine storm29,77,78. Unlike other innate immune cells, NKs are capable of memory-like properties—after exposure to haptens, viral antigens, or cytokine-induced cell memory—allowing the immune system to respond more effectively to a second pathogen infection79. NKs also contribute to influencing acquired immune cells (i.e., B and T cells) by secreting cytokines and chemokines such as interferon-γ (IFN-γ), TNF-α, and CCL380,81. Chuanxiong, a traditional CHM, promoted the cytotoxicity of NKs by upregulating the expression of NKG2D ligands (NKG2DLs), major histocompatibility complex class I-related chains A and B through its active ingredient tetramethypyrazine4.
Recently, anti-cancer immunotherapy targeting the innate immune system is concentrated on activating innate immune cells such as M1 macrophages, DCs, and NKs through chimeric antigen receptor-macrophages (CAR-M), DCs-based vaccines, and CAR-NK cell immunotherapy, respectively73,82,83. However, DCs-based immunotherapy may produce adverse side effects through tumor-associated/tumor specific CD8+/CD4+ T cell responses; and CAR-NK cell therapy present challenges in homing immune cells to tumor tissues and maintaining durable efficacy84. Immunomodulatory methods focused on repolarization of tumor-associated macrophages present difficulties with potentially converting macrophages in normal tissues into the proinflammatory M1 phenotype68. The discovery of CHMs provides a novel solution for traditional tumor immunotherapy drawbacks where extracted pharmacologically active ingredients can direct the innate immune system and further potentiate adaptive immune system responses.
2.2. Modulate the adaptive immune system
Adaptive immunity is the ability of the host to resist infection through exposure to invading pathogens or artificial vaccination, and consequently triggering a precise immune response to specific antigens primarily through T and B lymphocytes34,35. Active ingredients of CHMs demonstrate immunomodulatory activity and induce durable long-term immunological memory to resist tumor invasion and metastasis through effectively augmenting the infiltration and proliferation of adaptive immune cell types (including cytotoxic T lymphocytes, helper T cells, regulatory T cells, and B cells)30,53,85. T cells coordinate cellular immunity through cytotoxic T lymphocytes (CTLs, CD8+ T cells), helper T cells (Ths, CD4+ T cells), and regulatory T cells (Tregs); and these cells also undertake a vital supporting role in humoral immune system to promote the proliferation and differentiation of B cells64,86.
2.2.1. Modulate the T cells
CTLs directly destroy infected cells through secreting perforin, TNF-α and FASL-mediating antitumor cytotoxicity by initiating apoptosis of tumor cells47,48,87. Ths direct the immune process through an adjuvant role with predominant subtypes of Th1 and Th2; in which Th1 cells secrete cytokines IFN-γ and TNF-α that activate tumor cell surface death receptors and induce epitope spreading on antigen-presenting cells, and Th2 cells secrete cytokines IL-4, IL-5, and IL-10 that primarily induce humoral immune responses and exacerbate cancer progression through inhibiting IFN-γ secretion62,88. Notably, human cytokine activities are associated with the Th1/Th2 balance, and antigen-presenting cells can influence the direction of polarization of Ths54,55,89. CD4+CD25+Foxp3+ Tregs avoid excess immune activation from self-antigens, but further accelerate tumor proliferation by inducing immune resistance when infiltrated into the tumor microenvironment43,90,91. Active ingredients of CHMs have achieved promising results on tumor immunomodulation through interacting with tumor related T cells consisting of these different mechanisms of action. To identify the individual active ingredient contributing to CTLs cytotoxicity, 594 small molecules originating from CHMs were screened and atractylenolide I was identified to augment tumor-associated antigen presentation through binding to proteasome 26S subunit non-ATPase 4, increasing antigen-processing ability of immunoproteasome and consequently elevating the systemic immune response against tumors92. To regulate the balance of Th1 and Th2 subtypes, saikosaponin A purified from Bupleurum Radix-elicited the Th1 dominant response leading to an increase in CTLs and Ths infiltration within the tumor microenvironment to reduce breast cancer growth in vivo62 and ginsenoside purified from Panax ginseng C. A. Mey demonstrated antitumor effects through modulating the proportion of CTLs93. Extracts derived from Astragalus demonstrated a polarization of Th2 predominant cells to Th1 cells to elicit antitumor effects in patients with cervical cancer, increasing the populations of CD4+IFN-γ+ cell and CD4+IL-4+ cell in total CD4+ cells94. Additionally, the active ingredients of scutellarin from flavonoid could directly inhibit activation and expansion of Tregs by disrupting the interaction of tumor necrosis factor (TNF)-tumor necrosis factor receptor type II (TNFR2), subsequently inhibiting the phosphorylation of p38 MAPK, a downstream signaling component of TNFR2 expressed on Tregs, and down-regulating the activity of TNFR2+ Treg95.
2.2.2. Modulate the B cells
Naïve B cells directly recognize and clear tumor-associated antigens in a Ths-independent activation; whereas, B cells receptor-antigen and co-stimulation signal of CD40 and CD40L expressed on Ths are required to activate the humoral immunity response. In addition to antibody production, B cells can function as antigen presentation cells for activating cellular immune response and alter the response mode of T cells by secreting tumor-specific antibodies and inflammatory cytokines96. The two polysaccharide fractions, CDA-1A and CDA-3B, were derived from the cold-water extracts of the valuable CHMs Cistanche deserticola Y. C. Ma; and in vitro immunological testing showed that these polysaccharide fractions displayed potent bioactivity on B cells proliferation97.
2.3. Modulate the microenvironment signal molecule
The occurrence and development of tumors are affected by the dynamic network of stromal cells, immune cells, blood vessels, and signal molecules through positive and negative feedback interactions. Inflammatory cytokines dominate antitumor immunity, whereas inhibitory molecules (such as PD-1/PD-L1) hinder the infiltration and proliferation of immune cells to induce immune evasion54,58,98.
2.3.1. Modulate the cytokines
Of note, cytokines are pertinent signaling molecules that activate the systemic immune response, regulating interactions between cell populations within the immune system and between the immune system and surrounding cells. Cytokine levels can be used as an indicator for the assessment of tumor diagnosis, efficacy, and prognosis due to a tumor's pro-inflammatory and pro-angiogenic properties99. These molecules can be targeted to preferentially localize and activate anticancer immunity, in which the cytokines of interferon-α (IFN-α), interleukin-2 (IL-2) have been approved by the US Food and Drug Administration (FDA) in the United States for treatment of various malignant cancer types100. Despite their ability to elicit an anti-tumor immune response, cytokine therapies are often associated with poor patient response rates due to severe adverse side effects from non-antigen-specific toxicities101. CHMs extracted from nature with moderate medicinal property and multi-target effect have shown excellent efficacy on tumor immunomodulation28. Ginseng berry polysaccharide (GBPP), an immunostimulant extracted from natural medicine P. ginseng, significantly promoted the secretion of anti-melanoma cytokines (including IL-6, IL-12, IFN-γ, and TNF-α) by activated peritoneal macrophages in dose-dependent fashion, for a potential treatment of lung cancer model established by intravenously injected with B16-BL6 melanoma cells102.
2.3.2. Modulate the immune checkpoint
The interaction of immune cells and tumors within tumor microenvironment determines the amplitude and mechanism of immune response interactions; however, overexpressed immune checkpoint molecule restricts immune activation and can result in immune tolerance. The PD-1/PD-L1 immune checkpoint axis in which programmed cell death 1 (PD-1) binds to programmed cell death ligand 1 (PD-L1) on tumor cells to establish immunologic escape103. Blocking the PD-1/PD-L1 axis via biological agents, such as Nivolumab and Pembrolizumab, has limited clinical efficacy rates due to a high prevalence of immune-related adverse events in patients104. The natural medicine Platycodon grandiflorum is widely used in the field of biomedicine with immunomodulation, anti-inflammatory, anti-tumor, expectorant and cough, hypoglycemic, and anti-obesity effects. Recent investigation indicated P. grandiflorum effectively restricted the expression of PD-1 on CTLs within the tumor microenvironment, locally increasing the T cells responses while avoiding over-activation of systemic immunity in an in vivo non-small cell lung cancer model105. Combining active ingredients of CHMs with chemical or biological agents is a promising strategy to assist cancer immunotherapy. Mice treated with curcumin and sildenafil reduced the PD-L1 expression on CT26 tumors, and significantly improved tumor inhibition when combined with anti-PD-1 antibody or 5-flurouracil106. Therefore, the active ingredient of CHMs can be regarded as a neoadjuvant to enhance tumor immunotherapy and achieve amplified immunomodulatory effects in combination therapies31,50.
3. Nano-formulated active ingredients of CHMs for improving bioavailability
Despite evidence of CHMs possessing antitumor characteristics, active ingredients purified from CHMs may possess limited clinical applications due to undesirable pharmaceutical properties, such as hydrophobicity, poor stability, restricted circulation, poor targeting capabilities, and insufficient permeability107,108. The introduction of nanotechnology is expected to improve the active ingredient's bioavailability through increasing solubility and stability, extending half-life, optimizing targeting, and enhancing permeability. By engineering nano-formulated active ingredients of CHMs, these compounds can individually regulate host immune responses or be incorporated with chemotherapy, surgery and/or radiotherapy to maximize antitumor efficacy, proving novel clinical oncology treatments.
3.1. Increased solubility and stability
The poor solubility and stability of active ingredients in CHMs result in suboptimal bioavailability, which affects oral absorption and injectable administration. With the assistance of nanomaterials, active ingredients of CHMs can be loaded onto carrier materials in the form of absorption and/or encapsulation—to increase the drug's solubility and avoid direct exposure in the recipient. Polymeric nanomicellular systems for the delivery of baicalin, a hydrophobic active ingredient from the root of Scutellaria baicalensis Georgi, enhanced the compound's solubility and stability by encapsulating baicalin within a hydrophobic cavity constructed of conjugated stearic acid and pluronic F68. This nano-formulation exhibited monodisperse nano-sized particles with a high entrapment efficiency, that significantly mediated A549 human lung cancer cell cytotoxicity through modulating the chemical moieties of hydrophobic CHMs15.
3.2. Extended half-life
When exposed to systemic circulation, active ingredients of CHMs tend to be eliminated by immunosurveillance systems that recognize and clear exogenous substances, leading to an unfavorable half-life and poor bioavailability. Nano-formulated CHMs extend the half-life of active pharmacological agents by preventing the exogenous drug from being recognized by the immune system. To further extend the CHM's half-life, PEGylated nanocarriers have been used to alter the active pharmaceutical agent's metabolic properties, prolonging the retention and circulation time in physiological environments—thus promoting its aggregation in tumor target sites109,110. The piperine extracted from the seeds of Piperaceae has previously demonstrated adjuvant characteristics for breast cancer chemotherapy by eliciting reversing drug resistance and anti-metastatic bioactivities; however, piperidine's hydrophobicity leads to poor bioavailability at high concentrations111. Piperine-loaded aptamer-functionalized polyethylene glycol polylactide-co-glycolide nanoparticles (APT-P-PEG-PNP) effectively prolonged the retention of piperine in circulation and enhanced its antiproliferative activity against MCF-7 cells in combination with paclitaxel by inhibiting P-gp activity112.
3.3. Optimized targeting
Targeted drug delivery systems prolong and localize pharmacological compounds to specific cells, tissues, and organs in a dose-dependent manner. Although nano-drug delivery systems such as polymeric nanoparticles, liposomes, inorganic materials, and nanofibers demonstrate excellent targeting abilities in preclinical models with a size-controlled manner, these targeted drug delivery systems often result in negligible clinical outcomes113. According to the characteristics of tumor microenvironments, nano-formulated delivery systems can be optimized by modified ligands binding to receptors overexpressed on tumor cells such as: transferrin-receptors, epidermal growth factor receptors, and folate receptors. Alternately, nano-formulations can be functionalized as tumor microenvironment responsive delivery systems by introducing pH-sensitive, redox-sensitive, and enzyme-sensitive linkers to achieve on-demand drug release114. A targeted and redox-responsive nano-formulation containing camptothecin conjugated to lactose (CPT-S-S-LA) promoted the CPT accumulation in tumor cells through the interaction of lactose with sialoglycoprotein receptors on the hepatocellular carcinoma cells surface115. These interactions significantly enhanced the antitumor ability and alleviated the toxic side effects by promoting a responsive release of the drug as a response to high glutathione levels.
3.4. Enhanced permeability
The primary prerequisite for drug treatment is that sufficient active ingredients accumulate at target cells. However, active ingredients of CHMs encounter penetration obstacles in the tumor tissues due to high-density tumor cells, a dense extracellular matrix, and interstitial fluid pressure within the tumor microenvironment116. Engineering nanosized delivery systems with penetration-assisted peptides or multifunctional transformable properties can help increase the prevalence of drugs within the tumor microenvironment. A biomimetic, targeted nano-formulation of icariin functionalized with an internalized RGD peptide (iRGD, sequence c(CRGDK/RGPDC)) to enhance the water solubility and biocompatibility of icariin. The iRGD could bind to αvβ integrins expressed on the surface of tumor-associated endothelial cells and then generates CRGDK/R fragments, which further interacts with neuropilin-1 (NRP-1) receptors on tumor cells, eventually penetrating inside the tumor cells in the NRP-1-dependent endocytosis manner. Indeed, this nano-formulation improved tumor penetration in an A549 lung cancer cell model and increased the efficacy of icariin117. Enzyme-activated drug–protein conjugates have also been investigated to enhance the permeability of nanoparticle delivery systems across the tumor microenvironment. A membrane γ-glutamyl transpeptidase-triggered charge reversal CPT-polymer conjugate infiltrated the tumor microenvironment via caveolae-mediated endocytosis and transcytosis, significantly augmenting CPT-triggered tumor regression118.
4. Nano-formulated delivery of active ingredients from CHMs for cancer immunotherapy
Engineered nanomaterials can help protect the active ingredients from direct contact with blood by means of carrier adsorption, encapsulation, or conjugation, leading to increased drug efficacy119. Nano-formulated active ingredients of CHMs tend to enhance the retention, accumulation, and penetration of active ingredients in tumor sites, in addition to facilitating a controlled release of active ingredients in the tumor extracellular matrix or tumor cells. Notably, nanomaterials allow for synergistic treatment by loading multiple components, overcoming drug resistance, and activating immune responses through multiple pathways against tumors (Table 2)120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147.
Table 2.
Nano-formulated active ingredients originated from CHMs for cancer immunotherapy.
| Active ingredient | Carrier type | Loading method | Tumor model | Ref. |
|---|---|---|---|---|
| Astragaloside III | Mesoporous silica nanoparticle | Hydrophobic interaction | CT26 colon cancer | 120 |
| Angelica sinensis polysaccharide | Nanoparticle | Esterification reaction | MCF-7 human breast cancer | 121 |
| Astragalus polysaccharide | Nanoparticle | Esterification reaction | 4T1 breast cancer | 122 |
| Gold nanoparticle | Hydrophobic interaction | 4T1 breast cancer | 123 | |
| Celastrol | Polymeric nanoparticle | Hydrophobic interaction | D4M, BPD6 melanoma | 124 |
| Nanoemulsion | Hydrophobic interaction | B16F10 melanoma | 125 | |
| Curcumin | Nanofiber | Amidation reaction | LLC lung cancer | 126 |
| Liposome | Hydrophobic interaction | C26 colon carcinoma | 127 | |
| Camptothecin | Nanofiber | Esterification reaction | GL-261 brain cancer | 128 |
| Dihydrotanshinone I | Biomimetic nanoparticle | Hydrophobic interaction | Huh7, Hepa1-6 liver cancer | 129 |
| Epigallocatechin-3-O-gallate | Polymeric nanoparticle | Esterification reaction | 4T1 breast cancer | 130 |
| Ginsenoside Rg3 | Liposome | Hydrophobic interaction | C6 glioma | 131 |
| Ginsenoside Rg3, quercetin | Cyclodextrin nanoparticle | Hydrophobic interaction | CT26, HCT116 colorectal cancer | 132 |
| Ganoderma lucidum polysaccharide | Gold nanoparticle | Au-SH interaction | 4T1 breast cancer | 133 |
| Icaritin | Polymeric nanoparticle | Hydrophobic interaction | Huh7, Hepa1-6 liver cancer, B16 melanoma | 134 |
| Norcantharidin | Liposome | Hydrophobic interaction | H22 liver cancer | 135 |
| Puerarin | Nanoemulsion | Hydrophobic interaction | 4T1 breast cancer | 136 |
| Podophyllotoxin | Lipid nanoparticle | Amidation reaction | H460 lung cancer | 137 |
| Quercetin, alantolactone | Polymeric micelle | Hydrophobic interaction | CT26-FL3 colorectal cancer | 138 |
| Silybin | Liposome | Hydrophobic interaction | 4T1 breast cancer | 139 |
| Shikonin | Lactoferrin nanoparticle | Hydrophobic interaction | CT26 colon cancer | 140 |
| Liposome | Hydrophobic interaction | B16F10 melanoma | 141 | |
| Polymeric micelle | Hydrophobic interaction | CT26 colon cancer | 142 | |
| Silibinin | Polymeric nanoparticle | Hydrophobic interaction | 4T1 breast cancer | 143 |
| Salvianolic acid B | Liposome | Hydrophobic interaction | 4T1 breast cancer | 144 |
| Ursolic acid | Liposome | Hydrophobic interaction | 4T1 breast cancer | 145 |
| Excipient-free nanodrug | Hydrophobic, π–π stacking interactions | A549 lung cancer, Hela cervical cancer | 146 | |
| Ursolic acid, lentinan | Excipient-free nanodrug | Hydrogen bond, van der Waals force | CT26 colon cancer | 147 |
4.1. Polymer-assisted nano-formulation
4.1.1. Polymeric nanoparticles
Polymeric nanoparticles (PNPs) delivery systems consist of synthetic or naturally-occurring polymeric materials that agglomerate into nano-sized PNPs to load active ingredients in their inner core or covalently link functional groups on active ingredients121, 122, 123. By introducing targeting ligands or pH-sensitive linkers, PNPs can more effectively modulate the cancer immune environment120,124,130,137. A polymeric nanoparticle composed of poly lactic-co-glycolic acid-polyethylene glycol-aminoethyl anisamide (PLGA-PEG-AEAA)-encapsulated icaritin and doxorubicin was formulated for hepatocellular carcinoma treatment134. The icaritin moiety activated the autophagy pathway, potentiating tumor immunogenicity synergistically with doxorubicin, to serve as an immune activator (Fig. 2A).
Figure 2.
The polymeric nanoparticle delivery of active ingredient from CHMs to induce anticancer immune responses. (A) Schematic illustration of ICT + DOX NPs for provoking immunogenic cell death and activating adaptive immunity. Reprinted with the permission from Ref. 134. Copyright © 2020 American Chemical Society. (B) Schematic illustration of Comb-NP for targeting hepatocellular carcinoma cells and remodeling the immunosuppressive microenvironment. Reprinted with the permission from Ref. 129. Copyright © 2022 Elsevier. (C) Schematic illustration of FA-targeted co-formulation co-delivery of ginsenoside Rg3 and quercetin to exert synergic chemo-immunotherapy. Reprinted with the permission from Ref. 132. Copyright © 2022 Elsevier.
Ligand-receptor interactions direct the targeting of drug delivery systems, promoting drug enrichment in target sites and avoiding undesirable side effects on healthy tissues. To target hepatocellular carcinoma cells, a mannose-coated poly (d,l-lactic-co-glycolic acid) (PLGA)-based nanoparticle was designed to deliver two CHMs, plumbagin (PLB) and dihydrotanshinone I (DIH), as shown in Fig. 2B. This nano-formulation effectively increased the half-life and tumor enrichment capacity of CHMs, reprogramming the “cold-tumor” by inducing tumor immunogenic cell death and activating systemic immune responses129. Additionally, a folate-coated polyethylene glycol (PEG)-modified cyclodextrin nanoparticle was utilized to co-encapsulate natural medicines ginsenoside Rg3 and quercetin for cancer chemo-immunotherapy132. Quercetin activated reactive active oxygen species to enhance the ginsenoside Rg3-induced tumor immunogenicity, thus activating innate-adaptive immunity against tumor growth and prolonging survival rates in combination with PD-L1 antibodies (Fig. 2C).
4.1.2. Polymeric micelles
Polymeric micelles are promising drug delivery systems formed by the self-assembly of amphiphilic block copolymers in aqueous solutions above the critical micelle concentration148. Polymeric micelles possess unique core–shell architectures with a hydrophilic outer shell and a hydrophobic inner core, imparting multiple biological properties. The hydrophobic inner core serves as an ideal compartment for embedding lipophilic drugs, protecting active ingredients from degradation, and enhancing solubilization. Meanwhile, the hydrophilic exterior creates a tight protective shell that reduces protein and cell adhesion, prolongs blood circulation times, and increases the drug's half-life. Polymeric micelles consisted of FDA-approved polymers, TPGS and DSPE-PEG2000, were investigated for the codelivery of quercetin (originated from Inula racemosa Hook. F) and alantolactone (isolated from Inula helenium L.) with a specific drug ratio of 1:4. Combined with alantolactone, the quercetin strengthened the tumor immunogenicity and induced a tumor surveillance with durable memory138.
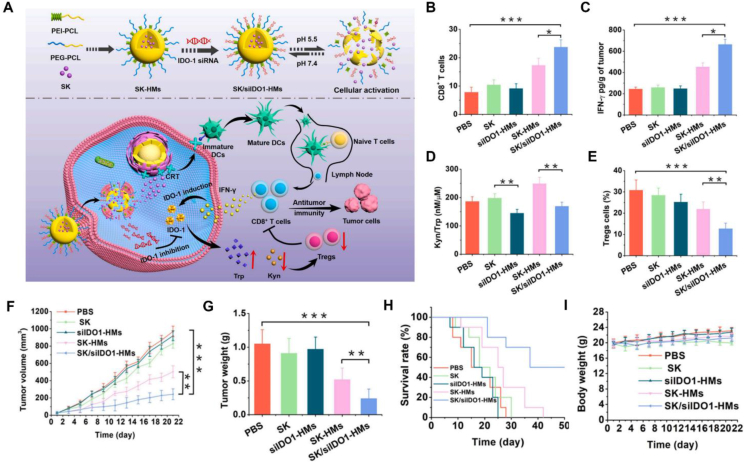
Hybrid micelles are also an encouraging strategy for maximizing cancer immunomodulatory potential through the co-delivery of therapeutic agents. Hybrid polymeric micelles co-delivering shikonin (SK, originated from the L. erythrorhizon plant) and indoleamine 2,3-dioxygenase-1 (IDO-1) knockdown siRNA (siIDO1) effectively induced anticancer immune response by remodeling the tumor microenvironment142. These hybrid polymeric micelles (SK/siIDO1-HMs) were prepared using polyethylene glycol-polycaprolactone (PEG-PCL) and polyethyleneimine-polycaprolactone (PEI-PCL), where PCL constituted the hydrophobic core to encapsulate SK while PEI with positive charges adsorbed the siIDO1 (Fig. 3A). The SK/siIDO1-HMs prolonged the circulation time to favor intratumoral accumulation such that SK moiety promoted DCs maturation and increased the proportions of infiltrating CTLs in tumor sites. The IFN-γ cytokines secreted by CTLs upregulated the expression of inhibitory molecule IDO1, leading to immune resistance (Fig. 3B–E). Following IDO pathway blockade by siIDO1, the SK/siIDO1-HMs displayed a remarkable anti-tumor efficacy in reducing tumor volume and extending survival rate (Fig. 3F–I).
Figure 3.
The polymeric micelles delivery of active ingredients from CHMs to induce anticancer immune responses. (A) Schematic illustration of the SK-siIDO1-HMs remolded tumor immunosuppressive microenvironment and enhanced antitumor immunity. (B) Quantitative measurement of immunofluorescence of intratumoral CD8+ T cells. (C) Detection of intratumoral IFN-γ secretion. (D) Evaluation of the IDO effect under corresponding treatments. (E) The infiltration of intratumoral Tregs. (F) The change of tumor volume. (G) Tumor weight following corresponding treatments. (H) Survival ratio curve. (I) Body weight in various treatments. Reprinted with the permission from Ref. 142. Copyright © 2021 Elsevier.
4.2. Lipid-assisted nano-formulations
4.2.1. Liposomes
Liposomal drug delivery systems are formed by phospholipid bilayer membranes encapsulating drug molecules; and these nanoparticles possess superior biocompatibility and lower immunogenicity due to their structural similarity to biological membranes127,149,150. Owing to its unique lipid bilayer structure, liposomes have various applications for delivery of both hydrophobic and hydrophilic molecules alike135,151. Their internal water cavity is suitable for loading hydrophilic drugs, while possessing lipophilic drugs between the bilayers of lipid molecules141. PEG-modified liposomes have been incorporated to entrap hydrophilic salvianolic acid B (which originated from S. miltiorrhiza Bunge) in the liposome's inner cavity to prolong its biological half-life time in vivo (Fig. 4A). When accumulated in tumor sites, salvianolic acid B reversed the tumor microenvironment through converted M2 macrophages into M1 macrophages and inhibited Tregs infiltration (Fig. 4B). Moreover, this nano-formulation improved the chemotherapeutic efficacy exerted by docetaxel-loaded PEG-modified liposomes144. Liposomes composed of hydrogenated soy phosphatidyl-choline, cholesterol and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] have been utilized to increase the solubility and control the release dynamics of a natural herb, ursolic acid (UA), for tumor microenvironment modulation145. The UA liposomes effectively ameliorated the tumor immunosuppressive state through down-regulating the percentage of Tregs and myeloid-derived suppressor cells residing in the tumor tissues, further strengthened the immune response against 4T1 triple negative breast cancer cells.
Figure 4.
The liposomal delivery of active ingredients from CHMs to induce anticancer immune responses. (A) Schematic diagram of PEG-SAB-Lip. (B) Schematic illustration of PEG-SAB-Lip for immune microenvironment modulation. Reprinted with the permission from Ref. 144. Copyright © 2022 Elsevier. (C) Schematic illustration of the multifunctional Rg3-PTX-LPs for glioma targeting chemo-immunotherapy. (D) Determination of tumor tissue-resident M1 and M2-type macrophages in tumor tissues. (E) In vivo survival ratios following corresponding treatments. Reprinted with the permission from Ref. 131. Copyright © 2021 Elsevier.
Plant-derived liposomes have been investigated to help improve the safety and fluidity of liposomes152. For example, ginsenoside Rg3, derived from P. ginseng roots, may serve as a substitute for cholesterol in liposomal drug delivery systems131. This molecule demonstrated brain cell-targeting capabilities through crossing the blood–brain barrier, which is attributed to the interaction of Rg3 glucosyl residues with glucose transporters located on cancer cells. The multifunctional Rg3-based liposomal system also exhibited synergic efficacy when combined with chemotherapeutic drugs (Fig. 4C). The paclitaxel-loaded Rg3-liposome significantly strengthened innate and adaptive immune responses through enhancing the M1/M2 ratio and expanded CTLs against glioma growth, resulting in prolonged median survival rates (Fig. 4D and E).
4.2.2. Nanoemulsions
Nanoemulsions are formed by water, oil, surfactant and co-surfactant in appropriate proportions to construct a low viscosity, thermodynamically stable, transparent and heterogeneous dispersion drug delivery system125. They are comprised of two incompatible phases: a discontinuous phase and continuous phase. Based on the composition and relative distribution of the dispersed and continuous phases, nanoemulsions can be classified as two-phase (O/W or W/O) or multi-phase (W/O/W). In addition to being an effective solubilizing carrier, nanoemulsions can also improve the bioavailability and control the release of insoluble active ingredients153,154. For example, puerarin nanoemulsions (nanoPue) utilize lecithin as the main emulsifier to improve the solubility of puerarin (Fig. 5A). The active ingredient of puerarin, an isoflavone derivative originating from the kudzu root, has the capacity to regulate the tumor stromal microenvironment. The nanoPue formulation significantly improved the CHM's solubility and therapeutic efficacy through remodeled stromal microenvironment, enhanced M1/M2 ratio, and reduced Tregs infiltration (Fig. 5B‒E). NanoPue can be treated as an adjuvant to paclitaxel and anti-PD-L1 antibodies, with demonstrated tumor inhibition and prolonged median survival rates in a 4T1 tumor model136.
Figure 5.
The nano-emulsion and inorganic nano-formulation delivery of active ingredients from CHMs to induce anticancer immune response. (A) Schematic diagram of the nano-puerarin regulated tumor microenvironment and boosted cancer immunotherapy. (B) The percentages of infiltrated CD4+ and (C) CD8+ T cells in tumor tissues. (D) The M1/M2 ratio in therapeutic and control groups. (E) The populations of Tregs in tumor tissues. Reprinted with the permission from Ref. 136. Copyright © 2020 Elsevier. (F) Schematic diagram of GLP-Au + DOX for activating DCs and memory T cells. (G) The percentages of CD4+CD44+ T cells under corresponding experimental groups. Reprinted with the permission from Ref. 133. Copyright © 2019 Elsevier.
4.3. Inorganic material-assisted nano-formulations
Inorganic drug delivery systems such as gold nanoparticles (AuNP), magnetic nanoparticles, and mesoporous silica nanoparticles demonstrate great potential in cancer immunotherapies due to their high surface area, size tunability, unique optical, and magnetic properties155. AuNP are considered to be the most widely used metal nanoparticles in the field of drug delivery due to their biocompatibility, unique optical properties, high surface area, and excellent surface modifiability156. These nanoparticles can load targeting ligands or drug molecules in various ways to facilitate drug release kinetics including electrostatic interactions, hydrophobic interactions, and covalent binding157. As shown in Fig. 5F, AuNPs were conjugated to the natural herbal Ganoderma lucidum polysaccharide (GLP) to elicit immunoregulatory functions to inhibit 4T1 tumor growth133. The GLP-Au nano-formulation promoted the GLP accumulation in tumor tissues and up-regulated the expression of CD80 and CD86 on DCs, thus inducing DCs maturation and T cells proliferation. Additionally, the GLP-Au nano-formulation increased the proportion of CD4+/CD44+ memory T cells in synergy with the chemotherapeutic doxorubicin (Fig. 5G). Thus, immunoreactive active ingredients of CHMs can be formulated with inorganic-materials for improving the compound's bioactivity; however, potential long-term toxicity and clearance rates of inorganic materials in vivo should be fully considered.
4.4. Other nano-formulations
4.4.1. Excipient-free nanodrugs
Biomaterial-assisted nano-formulations increase the drug's accumulation in target sites and prolong the drug's release behavior, thus avoiding unexpected side effects on healthy tissues. Unfortunately, this nano-formulation requires repeated dosing due to its insufficient drug-loading efficiency. To maximum the drug efficacy, nanodrug-based nanoparticles utilize self-assembly by prodrugs or amphiphilic drug–drug conjugates that lead to an increased drug-loading capacities and have the potential to release drugs as a response to a stimulus158. Recent studies indicated that the active ingredients of CHMs can spontaneously form stable excipient-free nanodrug structures under certain conditions, favorably reducing multiple dosing and improving biocompatibility159. Fig. 6A demonstrates an LNT-UA nanodrug composed of ursolic acid (UA) and lentinan (LNT) via hydrogen bond and van der Waals force147. This nanodrug without additional carrier material effectively improved the bioavailability of the naturally active ingredient UA, which initiated the immune response program to reverse the tumor immunosuppressive microenvironment. Combined with immune checkpoint inhibitor anti-CD47 antibody, this nanodrug reinforced systemic immunity against tumor growth and metastases as demonstrated in bilateral and spontaneous colorectal cancer models.
Figure 6.
The various nano-formulation delivery of active ingredients from CHMs to induce anticancer immune response. (A) Schematic diagram of excipient-free nanodrug LNT-UA for modulating macrophages and facilitated CD47 antibody-mediated anticancer immunity. Reprinted with the permission from Ref. 147. Copyright © 2022 Ivyspring International Publisher. (B) Schematic diagram of extracellular vesicles for modulating tumor-associated macrophages. Reprinted with the permission from Ref. 161. Copyright © 2019 BMJ Publishing Group Ltd. (C) Schematic diagram of diCPT-iRGD nanofibers for modulated innate-adaptive immune responses against tumors. Reprinted with the permission from Ref. 128. Copyright © 2020 Springer Nature.
4.4.2. Extracellular vesicles
Extracellular vesicles (EVs) within bilayer lipid membranes are nano-scaled membrane vesicles secreted by cells, containing genetic information and regulatory molecules, to modulate intracellular communication160. EVs are recognized as a natural drug delivery system with low immunogenicity, homologous targeting abilities, and high biocompatibility; and can serve as excellent carriers for drug delivery in cancer immunotherapies. Ginseng-derived EVs was extracted and purified from the roots of P. ginseng C. A. Mey; and demonstrated immunomodulatory mechanisms against melanoma through altering M2 macrophage polarization and skewing the M1/M2 rate towards the M1-like phenotype, which tend to associate with TLR4 and myeloid differentiation antigen 88 signaling pathways (Fig. 6B)161. Subsequently, the tumor tissues were enriched in M1 macrophages and T cells, enhancing the immune response in the tumor microenvironment, which validated the macrophage-dependent mechanism of ginseng-derived EVs on melanoma growth inhibition.
4.4.3. Nanofibers
Nanofibers have shown promising application prospects in the field of tissue engineering and drug delivery due to their high surface-to-volume ratio, high porosity, and low density. Currently, most nanofibers are prepared by synthetic polymers, which may pose safety and degradability issues126,162. Notably, peptides with excellent biocompatibility and degradability have the potential to form nanofiber structures through self-assembly processes. Nanofiber delivery systems, constructed with peptide–drug conjugates, enable drug self-delivery. For example, a series of peptide–drug conjugate platforms constructed with tumor penetration peptide iRGD and camptothecin (CPT) derived from the seeds or root bark of Camptotheca acuminata decne were investigated163, 164, 165. The peptide–drug conjugate could self-assemble into nanofibers within a positively charged aqueous environment, with the potential to exert the “drug delivery by drug” strategy through coating negatively charged medicines such as interferon genes (STING) agonist CDA (Fig. 6C)128. CPT-induced tumor cell death and resulted in the release of DNA fragments into the cytoplasm, thus initiating STING-dependent activity to transform the tumor immunosuppressive microenvironment into an immunostimulatory microenvironment. Notably, this nanofiber could entangle in the physiological environment and subsequently form a hydrogelator after charge screening.
5. Conclusions and future prospects
In this review, we briefly introduce the integration of nanomaterials and CHMs to construct nano-formulated active ingredients extracted from CHMs for cancer immunotherapy. CHMs exhibit multi-channel, multi-target, and multi-level immunomodulatory activities and their active ingredients may be a promising alternative to traditionally used chemical and biological agents. With the adjuvant of nanomaterials such as polymeric nanoparticles, liposomes, inorganic carriers, and nanofibers, the bioavailability and bioactivity of active ingredients from CHMs are significantly improved through an increased solubility and stability, extended half-life, optimized targeting abilities, and enhanced penetration139,140,143,146. The active ingredients, extracted from natural CHMs, exert multiple immune effects by editing immune response mechanisms from modulating innate and adaptive immune response systems, and tumor microenvironment signal molecules. Through these multiple pathways, these active ingredients establish tumor-specific immunity, inhibit tumor progression, and alleviate immune resistance.
Despite the potential advantages of active ingredients from CHMs, there are many challenges that can hamper clinical translation, such as concerns related to quality control, large-scale processing, reproducibility, biocompatibility, and biosafety166, 167, 168. CHMs have a multi-potency effect and the extracts determine the disease and quality of treatment. Due to its active ingredients being derived from natural materials, its purity is constrained by the quality of the raw material and the choice of purification reagents, thus precise control of the extraction and purification process is a prerequisite to ensure reproducibility and large-scale processing. Notably, nanomaterial-adjuvant active ingredients of CHMs can address undesirable pharmacological characteristics such as hydrophobicity, short half-life, poor tumor targeting, and penetration, favoring improved therapeutic efficacy and reduced toxic side effects42,57,63. Although promising in mice model, the large differences between patients and mice models limit the generalization of results from mice to patients. Therefore, the more representative animal models such as orthotopic xenografts, genetically engineered animal model, and patient-derived xenografts are encouraged to carefully evaluate therapeutic efficacy and monitor toxic side effects in preclinical trials. Importantly, the biosafety and compatibility in clinical applications must be considered. The therapeutic effect in the clinic is significantly attenuated by immune-related adverse events, involving immune-associated pneumonia, cardiotoxicity, digestive tract toxicity, etc., which may be due to the over-activation of the immune system by the active ingredients to attack healthy organs. Combination therapy is a promising approach through the co-administration of Immunotherapeutic agents. The contraindications, drug ratio, administration order present new challenges for immunotherapy. Moreover, active targeting of the site of active ingredients action is expected to reduce immune-related adverse events through conjugation of targeting ligands or modification of nanomaterials. To avoid the additional effect of carrier materials, optimizing nanomaterials characteristics or selecting authorized biomaterials to deliver active ingredients of CHMs are amenable to benefit patients and avoid excessive side effects from foreign carrier materials. Overall, integrating biocompatible nanomaterials and purifying active ingredients derived from CHMs is critical to construct nano-formulated active ingredients for novel cancer immunotherapy solutions.
Acknowledgments
This work is supported by National Key Research and Development Program of China (No. 2022YFC3501905); Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (No. 2060302); National Natural Science Foundation of China (NSFC, No. 82104076); Science and Technology Commission of Shanghai Municipal (STCSM, No. 22S21902400, China); and Medicine-Engineering joint foundation of Shanghai Jiao Tong University (No. YG2022QN025 and YG2022QN050, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Guangji Zhang, Email: zgjtcm@zcmu.edu.cn.
Qiang Zhang, Email: zqdodo@bjmu.edu.cn.
Feihu Wang, Email: fhwang21@sjtu.edu.cn.
Author contributions
Qi Shang and Wandong Liu collected the related papers, drafted the original manuscript. Faith Leslie, Jiapei Yang, Mingmei Guo, and Mingjiao Sun collected the related papers and revised the manuscript. Guangji Zhang, Qiang Zhang, and Feihu Wang proposed and outlined this review, revised and finalized the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Ruan S.B., Huang Y.Y., He M., Gao H.L. Advanced biomaterials for cell-specific modulation and restore of cancer immunotherapy. Adv Sci (Weinh) 2022;9 doi: 10.1002/advs.202200027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deets K.A., Vance R.E. Inflammasomes and adaptive immune responses. Nat Immunol. 2021;22:412–422. doi: 10.1038/s41590-021-00869-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.C., Feng L., Su J.Y., Cui L., Dan L., Yan J., et al. Polysaccharides from Epimedium koreanum Nakai with immunomodulatory activity and inhibitory effect on tumor growth in LLC-bearing mice. J Ethnopharmacol. 2017;207:8–18. doi: 10.1016/j.jep.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Luan Y., Liu J.L., Liu X.L., Xue X., Kong F., Sun C., et al. Tetramethypyrazine inhibits renal cell carcinoma cells through inhibition of NKG2D signaling pathways. Int J Oncol. 2016;49:1704–1712. doi: 10.3892/ijo.2016.3670. [DOI] [PubMed] [Google Scholar]
- 5.Wu X., Wang S.P., Lu J.R., Jing Y., Li M.X., Cao J.L., et al. Seeing the unseen of Chinese herbal medicine processing (Paozhi): advances in new perspectives. Chin Med. 2018;13:4. doi: 10.1186/s13020-018-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L.X., Liu Z.N., Ye J., Sha M., Qian H., Bu X.H., et al. Artesunate exerts an anti-immunosuppressive effect on cervical cancer by inhibiting PGE2 production and Foxp3 expression. Cell Biol Int. 2014;38:639–646. doi: 10.1002/cbin.10244. [DOI] [PubMed] [Google Scholar]
- 7.Bamodu O.A., Kuo K.T., Wang C.H., Huang W.C., Wu A.T.H., Tsai J.T., et al. Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients. 2019;11:2264. doi: 10.3390/nu11102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCune J.S. Rapid advances in immunotherapy to treat cancer. Clin Pharmacol Ther. 2018;103:540–544. doi: 10.1002/cpt.985. [DOI] [PubMed] [Google Scholar]
- 9.Yang C.Y., Fan S.M., Wang X., Liu W., Yang L., He B., et al. Optimize the combination regimen of trastuzumab and Nab-paclitaxel in HER2-positive tumors via modulating Caveolin-1 expression by lovastatin. Asian J Pharm Sci. 2022;17:697–712. doi: 10.1016/j.ajps.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning X., Wiraja C., Chew W.T.S., Fan C., Xu C. Transdermal delivery of Chinese herbal medicine extract using dissolvable microneedles for hypertrophic scar treatment. Acta Pharm Sin B. 2021;11:2937–2944. doi: 10.1016/j.apsb.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L.G., Peng X.C., Yu T.T., Xu H.Z., Han N., Yang X.X., et al. Dihydroartemisinin remodels macrophage into an M1 phenotype via ferroptosis-mediated DNA damage. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.949835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya S., Hossain D.M.S., Mohanty S., Sankar S.G., Chattopadhyay S., Banerjee S., et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7:306–315. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C.J., Chu C.Y., Huang L.H., Wang M.H., Sheu L.F., Yeh J.I., et al. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319:203–213. doi: 10.1016/j.canlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang K.L., Yu Y.C., Chen H.Y., Chiang Y.F., Ali M., Shieh T.M., et al. Recent advances in Glycyrrhiza glabra (Licorice)-containing herbs alleviating radiotherapy- and chemotherapy-induced adverse reactions in cancer treatment. Metabolites. 2022;12:535. doi: 10.3390/metabo12060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jangid A.K., Agraval H., Rai D.B., Jain P., Yadav U.C., Pooja D., et al. Baicalin encapsulating lipid-surfactant conjugate based nanomicelles: preparation, characterization and anticancer activity. Chem Phys Lipids. 2020;233 doi: 10.1016/j.chemphyslip.2020.104978. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Z.S., Yang Y.F., Yang Y.L., Zhang Y., Yue Z.S., Pan Z.Y., et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed Pharmacother. 2017;96:378–383. doi: 10.1016/j.biopha.2017.09.129. [DOI] [PubMed] [Google Scholar]
- 17.Ri M.H., Ma J., Jin X. Development of natural products for anti-PD-1/PD-L1 immunotherapy against cancer. J Ethnopharmacol. 2021;281 doi: 10.1016/j.jep.2021.114370. [DOI] [PubMed] [Google Scholar]
- 18.Liu X.Y., Ding Y.X., Zhao B.J., Liu Y.Y., Luo S.L., Wu J.Y., et al. In vitro and in vivo evaluation of puerarin-loaded pegylated mesoporous silica nanoparticles. Drug Dev Ind Pharm. 2016;42:2031–2037. doi: 10.1080/03639045.2016.1190742. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y.P., Han S.P., Zheng H.Y., Ma R., Ping Y.T., Zou J.F., et al. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int J Nanomedicine. 2018;13:5937–5952. doi: 10.2147/IJN.S175418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi F., Zhang Y.T., Yang G., Guo T., Feng N.P. Preparation of a micro/nanotechnology based multi-unit drug delivery system for a Chinese medicine Niuhuang Xingxiao Wan and assessment of its antitumor efficacy. Int J Pharm. 2015;492:244–247. doi: 10.1016/j.ijpharm.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Wei D.H., Yang H., Zhang Y., Zhang X.H., Wang J., Wu X.L., et al. Nano-traditional Chinese medicine: a promising strategy and its recent advances. J Mater Chem B. 2022;10:2973–2994. doi: 10.1039/d2tb00225f. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y., Xiong J.Y., Sun X.Y., Gao H.L. Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Acta Pharm Sin B. 2022;12:4327–4347. doi: 10.1016/j.apsb.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhamad N., Plengsuriyakarn T., Na-Bangchang K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: a systematic review. Int J Nanomedicine. 2018;13:3921–3935. doi: 10.2147/IJN.S165210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang N., Zhang Y., Liu H.B., Wang A.D., Ren T.Y., Gou J.X., et al. Toxicity reduction and efficacy promotion of doxorubicin in the treatment of breast tumors assisted by enhanced oral absorption of curcumin-loaded lipid-polyester mixed nanoparticles. Mol Pharm. 2020;17:4533–4547. doi: 10.1021/acs.molpharmaceut.0c00718. [DOI] [PubMed] [Google Scholar]
- 25.Ali S., Kjeken R., Niederlaender C., Markey G., Saunders T.S., Opsata M., et al. The European medicines agency review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist. 2020;25:e321–e327. doi: 10.1634/theoncologist.2019-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoro A., Moskowitz A.J., Ferrari S., Carlo-Stella C., Lisano J.M., Francis S., et al. Nivolumab combined with brentuximab vedotin for relapsed/refractory mediastinal gray zone lymphoma. Blood. 2023:2780–2783. doi: 10.1182/blood.2022017951. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Hu K.L., Di L.Q., Wang P.L., Liu Z.D., Zhang J.M., et al. Traditional herbal medicine and nanomedicine: converging disciplines to improve therapeutic efficacy and human health. Adv Drug Deliv Rev. 2021;178 doi: 10.1016/j.addr.2021.113964. [DOI] [PubMed] [Google Scholar]
- 28.Cui C., Feng H.L., Shi X.L., Wang Y.Z., Feng Z.Y., Liu J.L., et al. Artesunate down-regulates immunosuppression from colorectal cancer Colon26 and RKO cells in vitro by decreasing transforming growth factor β1 and interleukin-10. Int Immunopharmacol. 2015;27:110–121. doi: 10.1016/j.intimp.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.H., Lee S.W., Park H.J., Lee S.H., Im W.K., Kim Y.D., et al. Anti-cancer activity of Angelica gigas by increasing immune response and stimulating natural killer and natural killer T cells. BMC Complement Altern Med. 2018;18:218. doi: 10.1186/s12906-018-2277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T.H., Jia M., Meng J., Wu H., Mei Q.B. Immunomodulatory activity of polysaccharide isolated from Angelica sinensis. Int J Biol Macromol. 2006;39:179–184. doi: 10.1016/j.ijbiomac.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Chang H.L., Kuo Y.H., Wu L.H., Chang C.M., Cheng K.J., Tyan Y.C., et al. The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int J Med Sci. 2020;17:939–945. doi: 10.7150/ijms.42978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Z., Li Y.Y., Li Y., Zhang J.H., Li M., Ji L.S., et al. Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu R., Yu F.J., Wu W.Q., Liu J., Li J., Guo F.Y., et al. Bufalin enhances the killing efficacy of NK cells against hepatocellular carcinoma by inhibiting MICA shedding. Int Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.108195. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Liu X.J., Zhang N., Yin M.X., Dong J.W., Zeng Q.X., et al. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm Sin B. 2020;10:2299–2312. doi: 10.1016/j.apsb.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao F., Liu L., Luo E., Hu J. Curcumin enhances anti-tumor immune response in tongue squamous cell carcinoma. Arch Oral Biol. 2018;92:32–37. doi: 10.1016/j.archoralbio.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Liu S., Han Z., Trivett A.L., Lin H.S., Hannifin S., Yang D., et al. Cryptotanshinone has curative dual anti-proliferative and immunotherapeutic effects on mouse lewis lung carcinoma. Cancer Immunol Immunother. 2019;68:1059–1071. doi: 10.1007/s00262-019-02326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui L.Y., Yang G.D., Ye J.N., Yao Y.N., Lu G.H., Chen J.J., et al. Dioscin elicits anti-tumour immunity by inhibiting macrophage M2 polarization via JNK and STAT3 pathways in lung cancer. J Cell Mol Med. 2020;24:9217–9230. doi: 10.1111/jcmm.15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang J.Y., Lee J.K., Jeon Y.K., Kim C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravindran M.D., Li Y., Yamauchi T., Osborne D.G., Vaddi P.K., Wempe M.F., et al. EGCG inhibits tumor growth in melanoma by targeting JAK-STAT signaling and its downstream PD-L1/PD-L2-PD1 axis in tumors and enhancing cytotoxic T-cell responses. Pharmaceuticals (Basel) 2021;14:1081. doi: 10.3390/ph14111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H.L., Huang N., Zhu W.K., Wu J.C., Yang X.H., Teng W.J., et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer. 2018;18:579. doi: 10.1186/s12885-018-4299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M., Yan S.J., Zhang H.T., Li N., Liu T., Zhang Y.L., et al. Ginsenoside Rh2 enhances the antitumor immunological response of a melanoma mice model. Oncol Lett. 2017;13:681–685. doi: 10.3892/ol.2016.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C.Z., Hou L.F., Wan J.Y., Yao H.Q., Yuan J.B., Zeng J.X., et al. Ginseng berry polysaccharides on inflammation-associated colon cancer: inhibiting T-cell differentiation, promoting apoptosis, and enhancing the effects of 5-fluorouracil. J Ginseng Res. 2020;44:282–290. doi: 10.1016/j.jgr.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin M.S., Hwang S.H., Yoon T.J., Kim S.H., Shin K.S. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation. Int J Biol Macromol. 2017;103:1327–1333. doi: 10.1016/j.ijbiomac.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 44.Wang C.L., Lu C.Y., Hsueh Y.C., Liu W.H., Chen C.J. Activation of antitumor immune responses by Ganoderma formosanum polysaccharides in tumor-bearing mice. Appl Microbiol Biotechnol. 2014;98:9389–9398. doi: 10.1007/s00253-014-6027-6. [DOI] [PubMed] [Google Scholar]
- 45.Zheng X., Li D.H., Li J.X., Wang B.T., Zhang L.Q., Yuan X.F., et al. Optimization of the process for purifying icariin from herba epimedii by macroporous resin and the regulatory role of icariin in the tumor immune microenvironment. Biomed Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109275. [DOI] [PubMed] [Google Scholar]
- 46.Tian L., Wang S., Jiang S., Liu Z.Y., Wan X.Q., Yang C.C., et al. Luteolin as an adjuvant effectively enhances CTL anti-tumor response in B16F10 mouse model. Int Immunopharmacol. 2021;94 doi: 10.1016/j.intimp.2021.107441. [DOI] [PubMed] [Google Scholar]
- 47.Mushiake H., Tsunoda T., Nukatsuka M., Shimao K., Fukushima M., Tahara H. Dendritic cells might be one of key factors for eliciting antitumor effect by chemoimmunotherapy in vivo. Cancer Immunol Immunother. 2005;54:120–128. doi: 10.1007/s00262-004-0585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun M., Bu R.G., Zhang B., Cao Y.M., Liu C.Y., Zhao W.Y. Lentinan inhibits tumor progression by immunomodulation in a mouse model of bladder cancer. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420946823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W., Liu M.X., Wang Y., Yang T., Li D.S., Ding F., et al. Lycium barbarum polysaccharide promotes maturation of dendritic cell via notch signaling and strengthens dendritic cell mediated T lymphocyte cytotoxicity on colon cancer cell CT26-WT. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/2305683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng L., Xiao X., Liu J., Wang J.Y., Zhang N., Bing T., et al. Immunomodulatory effects of lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules. 2020;25:1351. doi: 10.3390/molecules25061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu S., Gao Y., Huang X.L., Wang X.H. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci. 2014;10:415–425. doi: 10.7150/ijbs.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mo L.J., Zhang X.J., Shi X.J., Wei L.L., Zheng D.P., Li H.W., et al. Norcantharidin enhances antitumor immunity of GM-CSF prostate cancer cells vaccine by inducing apoptosis of regulatory T cells. Cancer Sci. 2018;109:2109–2118. doi: 10.1111/cas.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q.J., Zheng X.C., Wang T., Zhang T.Y. Effects of oridonin on immune cells, Th1/Th2 balance and the expression of BLys in the spleens of broiler chickens challenged with Salmonella pullorum. Res Vet Sci. 2018;119:262–267. doi: 10.1016/j.rvsc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q.F., Huang H.D., Zheng F., Liu H.Z., Qiu F.F., Chen Y.C., et al. Resveratrol exerts antitumor effects by downregulating CD8+CD122+ Tregs in murine hepatocellular carcinoma. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1829346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han X., Zhao N., Zhu W.W., Wang J., Liu B.X., Teng Y. Resveratrol attenuates TNBC lung metastasis by down-regulating PD-1 expression on pulmonary T cells and converting macrophages to M1 phenotype in a murine tumor model. Cell Immunol. 2021;368 doi: 10.1016/j.cellimm.2021.104423. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Kwak M., Lee P.C., Jin J.O. Rehmannia glutinosa polysaccharide promoted activation of human dendritic cells. Int J Biol Macromol. 2018;116:232–238. doi: 10.1016/j.ijbiomac.2018.04.144. [DOI] [PubMed] [Google Scholar]
- 57.Xu L., Zhang W., Zeng L., Jin J.O. Rehmannia glutinosa polysaccharide induced an anti-cancer effect by activating natural killer cells. Int J Biol Macromol. 2017;105:680–685. doi: 10.1016/j.ijbiomac.2017.07.090. [DOI] [PubMed] [Google Scholar]
- 58.Peng F., Xiong L., Peng C. (–)-Sativan inhibits tumor development and regulates miR-200c/PD-L1 in triple negative breast cancer cells. Front Pharmacol. 2020;11:251. doi: 10.3389/fphar.2020.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin T.J., Lin H.T., Chang W.T., Mitapalli S.P., Hsiao P.W., Yin S.Y., et al. Shikonin-enhanced cell immunogenicity of tumor vaccine is mediated by the differential effects of DAMP components. Mol Cancer. 2015;14:174. doi: 10.1186/s12943-015-0435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y., Lu H.L., Gu Y.C., Ning Z.Q., Cao T.H., Chen C., et al. Enhancement of NK cells proliferation and function by shikonin. Immunopharmacol Immunotoxicol. 2017;39:124–130. doi: 10.1080/08923973.2017.1299174. [DOI] [PubMed] [Google Scholar]
- 61.Yin S.S., Jin W.K., Qiu Y.L., Fu L.L., Wang T., Yu H.Y. Solamargine induces hepatocellular carcinoma cell apoptosis and autophagy via inhibiting LIF/miR-192-5p/CYR61/Akt signaling pathways and eliciting immunostimulatory tumor microenvironment. J Hematol Oncol. 2022;15:32. doi: 10.1186/s13045-022-01248-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao X., Liu J.Y., Ge S.S., Chen C., Li S., Wu X.Y., et al. Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol. 2019;10:624. doi: 10.3389/fphar.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu B., Zhang H.Q., Li J., Lu C., Chen G., Zhang G., et al. Triptolide downregulates Treg cells and the level of IL-10, TGF-beta, and VEGF in melanoma-bearing mice. Planta Med. 2013;79:1401–1407. doi: 10.1055/s-0033-1350708. [DOI] [PubMed] [Google Scholar]
- 64.Kuo C.S., Yang C.Y., Lin C.K., Lin G.J., Sytwu H.K., Chen Y.W. Triptolide suppresses oral cancer cell PD-L1 expression in the interferon-gamma-modulated microenvironment in vitro, in vivo, and in clinical patients. Biomed Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111057. [DOI] [PubMed] [Google Scholar]
- 65.Yu X.M., Xu M.Y., Li N., Li Z.J., Li H., Shao S.J., et al. Β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem Biophys Res Commun. 2017;490:514–520. doi: 10.1016/j.bbrc.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann-Klemd A.M., Reinhardt J.K., Morath A., Schamel W.W., Steinberger P., Leitner J., et al. Immunosuppressive activity of artemisia argyi extract and isolated compounds. Front Pharmacol. 2020;11:402. doi: 10.3389/fphar.2020.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daza J., Charap A., Wiklund P.N., Sfakianos J.P. Role of the innate immune system in the development, progression, and therapeutic response of bladder cancer. Eur Urol Focus. 2020;6:650–652. doi: 10.1016/j.euf.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 68.Anderson N.R., Minutolo N.G., Gill S., Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–1208. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 69.Wang S.L., Liu G.H., Li Y.R., Pan Y.B. Metabolic reprogramming induces macrophage polarization in the tumor microenvironment. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.840029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehla K., Singh P.K. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. 2019;5:822–834. doi: 10.1016/j.trecan.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu C.P., He D.Y., Zhang S.H., Chen H.Q., Zhao J., Li X., et al. Homogeneous polyporus polysaccharide inhibit bladder cancer by resetting tumor-associated macrophages toward M1 through NF-kappaB/NLRP3 signaling. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.839460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stockwin L.H., McGonagle D., Martin I.G., Blair G.E. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol Cell Biol. 2000;78:91–102. doi: 10.1046/j.1440-1711.2000.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabado R.L., Balan S., Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia G.Y., Shao X.Y., Zhao R., Zhang T., Zhou X.C., Yang Y., et al. Portulaca oleracea L. polysaccharides enhance the immune efficacy of dendritic cell vaccine for breast cancer. Food Funct. 2021;12:4046–4059. doi: 10.1039/d0fo02522d. [DOI] [PubMed] [Google Scholar]
- 75.Raskov H., Orhan A., Salanti A., Gaggar S., Gogenur I. Natural killer cells in cancer and cancer immunotherapy. Cancer Lett. 2021;520:233–242. doi: 10.1016/j.canlet.2021.07.032. [DOI] [PubMed] [Google Scholar]
- 76.Malmberg K.J., Carlsten M., Bjorklund A., Sohlberg E., Bryceson Y.T., Ljunggren H.G. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Bjorkstrom N.K., Ljunggren H.G., Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16:310–320. doi: 10.1038/nri.2016.34. [DOI] [PubMed] [Google Scholar]
- 78.Li Y., Hermanson D.L., Moriarity B.S., Kaufman D.S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–192. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cerwenka A., Lanier L.L. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- 80.Maskalenko N.A., Zhigarev D., Campbell K.S. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discov. 2022;21:559–577. doi: 10.1038/s41573-022-00413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Demaria O., Gauthier L., Debroas G., Vivier E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol. 2021;51:1934–1942. doi: 10.1002/eji.202048953. [DOI] [PubMed] [Google Scholar]
- 82.Gong Y., Klein Wolterink R.G.J., Wang J., Bos G.M.J., Germeraad W.T.V. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73. doi: 10.1186/s13045-021-01083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujii S.I., Shimizu K. Immune networks and therapeutic targeting of iNKT cells in cancer. Trends Immunol. 2019;40:984–997. doi: 10.1016/j.it.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Laureano R.S., Sprooten J., Vanmeerbeerk I., Borras D.M., Govaerts J., Naulaerts S., et al. Trial watch: dendritic cell (DC)-based immunotherapy for cancer. Oncoimmunology. 2022;11 doi: 10.1080/2162402X.2022.2096363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mulder W.J.M., Ochando J., Joosten L.A.B., Fayad Z.A., Netea M.G. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18:553–566. doi: 10.1038/s41573-019-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang S.H., Sun J.W., Chen K., Ma P.W., Lei Q., Xing S.J., et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 2021;19:140. doi: 10.1186/s12916-021-02006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J.Q., Ye Y.Q., Cai L.L. Supramolecular attack particle: the way cytotoxic T lymphocytes kill target cells. Signal Transduct Target Ther. 2020;5:210. doi: 10.1038/s41392-020-00319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ostroumov D., Fekete-Drimusz N., Saborowski M., Kuhnel F., Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75:689–713. doi: 10.1007/s00018-017-2686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 90.Xu P.P., Fan W., Zhang Z., Wang J., Wang P., Li Y.R., et al. The clinicopathological and prognostic implications of Foxp3+ regulatory T cells in patients with colorectal cancer: a meta-analysis. Front Physiol. 2017;8:950. doi: 10.3389/fphys.2017.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H.C., Jeught K.V.D., Zhou Z.L., Zhang L., Yu T., Sun Y.F., et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J Clin Invest. 2021;131 doi: 10.1172/JCI146832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Z.M., Shao Z.J., Qu D., Huo X.H., Hua M., Chen J.B., et al. Transformation mechanism of rare ginsenosides in american ginseng by different processing methods and antitumour effects. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.833859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu Y.J., Li L., Gong S.X. Regulatory effect of astragalus injection on Th1/Th2 cell function in patients with cervical cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:1157–1159. [PubMed] [Google Scholar]
- 95.Chen S., Li R., Chen Y., Chou C.K., Zhang Z., Yang Y., et al. Scutellarin enhances anti-tumor immune responses by reducing TNFR2-expressing CD4+Foxp3+ regulatory T cells. Biomed Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113187. [DOI] [PubMed] [Google Scholar]
- 96.Ghosh D., Jiang W., Mukhopadhyay D., Mellins E.D. New insights into B cells as antigen presenting cells. Curr Opin Immunol. 2021;70:129–137. doi: 10.1016/j.coi.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 97.Dong Q., Yao J., Fang J.N., Ding K. Structural characterization and immunological activity of two cold-water extractable polysaccharides from Cistanche deserticola Y. C. Ma. Carbohydr Res. 2007;342:1343–1349. doi: 10.1016/j.carres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y.L., Zhang X.Y., Wang Y.Y., Zhao W.J., Li H.L., Zhang L.X., et al. Application of immune checkpoint targets in the anti-tumor novel drugs and traditional Chinese medicine development. Acta Pharm Sin B. 2021;11:2957–2972. doi: 10.1016/j.apsb.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neri D., Sondel P.M. Immunocytokines for cancer treatment: past, present and future. Curr Opin Immunol. 2016;40:96–102. doi: 10.1016/j.coi.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solinas C., Chanza N.M., Awada A., Scartozzi M. The immune infiltrate in prostate, bladder and testicular tumors: an old friend for new challenges. Cancer Treat Rev. 2017;53:138–145. doi: 10.1016/j.ctrv.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 101.Berraondo P., Sanmamed M.F., Ochoa M.C., Etxeberria I., Aznar M.A., Perez-Gracia J.L., et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee D.Y., Park C.W., Lee S.J., Park H.R., Kim S.H., Son S.U., et al. Anti-cancer effects of Panax ginseng berry polysaccharides via activation of immune-related cells. Front Pharmacol. 2019;10:1411. doi: 10.3389/fphar.2019.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nixon B.G., Li M.O. Satb1: restraining PD1 and T cell exhaustion. Immunity. 2017;46:3–5. doi: 10.1016/j.immuni.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 104.Hassel J.C., Heinzerling L., Aberle J., Bahr O., Eigentler T.K., Grimm M.O., et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. doi: 10.1016/j.ctrv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Yang R.J., Pei T.L., Huang R.F., Xiao Y., Yan J.N., Zhu J.L., et al. Platycodon grandiflorum triggers antitumor immunity by restricting PD-1 expression of CD8+ T cells in local tumor microenvironment. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.774440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dent P., Booth L., Roberts J.L., Poklepovic A., Hancock J.F. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J Cell Physiol. 2020;235:6862–6874. doi: 10.1002/jcp.29580. [DOI] [PubMed] [Google Scholar]
- 107.Wu H., Zhong Q.X., Zhong R.L., Huang H.C., Xia Z., Ke Z.C., et al. Preparation and antitumor evaluation of self-assembling oleanolic acid-loaded pluronic P105/d-alpha-tocopheryl polyethylene glycol succinate mixed micelles for non-small-cell lung cancer treatment. Int J Nanomedicine. 2016;11:6337–6352. doi: 10.2147/IJN.S119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma Z.W., Wang N., He H.B., Tang X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J Control Release. 2019;316:359–380. doi: 10.1016/j.jconrel.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 109.Iyer A.K., Khaled G., Fang J., Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Fan W.F., Peng H.X., Yu Z., Wang L.T., He H.S., Ma Y.H., et al. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm Sin B. 2022;12:2479–2493. doi: 10.1016/j.apsb.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hong C.W., Chin S., Pang S.C., Kok K.Y. Synthesis and characterisation of piperine-loaded starch nanoparticles. J Phys Sci. 2020;31:57–68. [Google Scholar]
- 112.Pachauri M., Gupta E.D., Ghosh P.C. Piperine loaded PEG-PLGA nanoparticles: preparation, characterization and targeted delivery for adjuvant breast cancer chemotherapy. J Drug Deliv Sci Technol. 2015;29:269–282. [Google Scholar]
- 113.Meng H., Leong W., Leong K.W., Chen C., Zhao Y. Walking the line: the fate of nanomaterials at biological barriers. Biomaterials. 2018;174:41–53. doi: 10.1016/j.biomaterials.2018.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng Z., Li M.Y., Dey R., Chen Y.H. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu L., Li B., Lin C.C., Li K., Liu G.H., Xia Z.Z.L., et al. Redox-responsive amphiphilic camptothecin prodrug nanoparticles for targeted liver tumor therapy. J Mater Chem B. 2020;8:3918–3928. doi: 10.1039/d0tb00285b. [DOI] [PubMed] [Google Scholar]
- 116.Ding J.X., Chen J.J., Gao L.Q., Jiang Z.Y., Zhang Y., Li M.Q., et al. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29 [Google Scholar]
- 117.Ji Y., Zhang Z.T., Hou W.J., Wu M., Wu H.S., Hu N., et al. Enhanced antitumor effect of icariin nanoparticles coated with iRGD functionalized erythrocyte membrane. Eur J Pharmacol. 2022;931 doi: 10.1016/j.ejphar.2022.175225. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Q., Shao S.Q., Wang J.Q., Xu C.H., Xiang J.J., Piao Y., et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 119.Akakuru O.U., Zhang Z., Iqbal M.Z., Zhu C., Zhang Y., Wu A. Chemotherapeutic nanomaterials in tumor boundary delineation: prospects for effective tumor treatment. Acta Pharm Sin B. 2022;12:2640–2657. doi: 10.1016/j.apsb.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu X.L., Yang H., Chen X.M., Gao J.X., Duan Y., Wei D.H., et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120654. [DOI] [PubMed] [Google Scholar]
- 121.Wang M.Z., He X., Yu Z., Wu H., Yang T.H. A nano drug delivery system based on Angelica sinensis polysaccharide for combination of chemotherapy and immunotherapy. Molecules. 2020;25:3096. doi: 10.3390/molecules25133096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pang G.B., Chen C., Liu Y., Jiang T.Y., Yu H., Wu Y.X., et al. Bioactive polysaccharide nanoparticles improve radiation-induced abscopal effect through manipulation of dendritic cells. ACS Appl Mater Interfaces. 2019;11:42661–42670. doi: 10.1021/acsami.9b16814. [DOI] [PubMed] [Google Scholar]
- 123.Xiong J., Jiang B.L., Luo Y., Zou J.Z., Gao X., Xu D., et al. Multifunctional nanoparticles encapsulating Astragalus polysaccharide and gold nanorods in combination with focused ultrasound for the treatment of breast cancer. Int J Nanomedicine. 2020;15:4151–4169. doi: 10.2147/IJN.S246447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Q., Chen F.Q., Hou L., Shen L.M., Zhang X.Q., Wang D.G., et al. Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma. ACS Nano. 2018;12:7812–7825. doi: 10.1021/acsnano.8b01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qiu N.S., Liu Y., Liu Q., Chen Y.Z., Shen L.M., Hu M.Y., et al. Celastrol nanoemulsion induces immunogenicity and downregulates PD-L1 to boost abscopal effect in melanoma therapy. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2020.120604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang T.T., Wang J., Jiang H., Ni M.N., Zou Y.F., Chen Y.L., et al. Targeted regulation of tumor microenvironment through the inhibition of MDSCs by curcumin loaded self-assembled nano-filaments. Mater Today Bio. 2022;15 doi: 10.1016/j.mtbio.2022.100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sesarman A., Tefas L., Sylvester B., Licarete E., Rauca V., Luput L., et al. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Deliv Transl Res. 2019;9:260–272. doi: 10.1007/s13346-018-00598-8. [DOI] [PubMed] [Google Scholar]
- 128.Wang F.H., Su H., Xu D.Q., Dai W.B., Zhang W.J., Wang Z.Y., et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat Biomed Eng. 2020;4:1090–1101. doi: 10.1038/s41551-020-0597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Han S.L., Bi S.N., Guo T.T., Sun D.D., Zou Y.F., Wang L.Z., et al. Nano co-delivery of plumbagin and dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J Control Release. 2022;348:250–263. doi: 10.1016/j.jconrel.2022.05.057. [DOI] [PubMed] [Google Scholar]
- 130.Sun X.Y., Zhang J.L., Xiu J.Y., Zhao X.F., Yang C.R., Li D., et al. A phenolic based tumor-permeated nano-framework for immunogenic cell death induction combined with PD-L1 immune checkpoint blockade. Biomater Sci. 2022;10:3808–3822. doi: 10.1039/d2bm00455k. [DOI] [PubMed] [Google Scholar]
- 131.Zhu Y., Liang J.M., Gao C.F., Wang A.N., Xia J.X., Hong C., et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J Control Release. 2021;330:641–657. doi: 10.1016/j.jconrel.2020.12.036. [DOI] [PubMed] [Google Scholar]
- 132.Sun D.D., Zou Y.F., Song L., Han S.L., Yang H., Chu D., et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sin B. 2022;12:378–393. doi: 10.1016/j.apsb.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang S.L., Pang G.B., Chen C., Qin J.Z., Yu H., Liu Y.M., et al. Effective cancer immunotherapy by ganoderma lucidum polysaccharide-gold nanocomposites through dendritic cell activation and memory T cell response. Carbohydr Polym. 2019;205:192–202. doi: 10.1016/j.carbpol.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 134.Yu Z., Guo J.F., Hu M.Y., Gao Y.Q., Huang L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano. 2020;14:4816–4828. doi: 10.1021/acsnano.0c00708. [DOI] [PubMed] [Google Scholar]
- 135.Liu Z.X., Zhao L.X., Liu H., Dong N., Zhou N., Zhang Y., et al. Norcantharidin liposome emulsion hybrid delivery system enhances PD-1/PD-L1 immunotherapy by agonizing the non-canonical NF-kappaB pathway. Int J Pharm. 2022;628 doi: 10.1016/j.ijpharm.2022.122361. [DOI] [PubMed] [Google Scholar]
- 136.Xu H., Hu M.Y., Liu M.R., An S., Guan K.Y., Wang M.L., et al. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials. 2020;235 doi: 10.1016/j.biomaterials.2020.119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang R., Zhao Y.N., Huang Z.L., Zhou Y.X., Wang W., Xuan Y., et al. Self-assembly of podophyllotoxin-loaded lipid bilayer nanoparticles for highly effective chemotherapy and immunotherapy via downregulation of programmed cell death ligand 1 production. ACS Nano. 2022;16:3943–3954. doi: 10.1021/acsnano.1c09391. [DOI] [PubMed] [Google Scholar]
- 138.Zhang J., Shen L.M., Li X., Song W.T., Liu Y., Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13:12511–12524. doi: 10.1021/acsnano.9b02875. [DOI] [PubMed] [Google Scholar]
- 139.Wu S.Y., Liu D., Li W.P., Song B.H., Chen C.L., Chen D.W., et al. Enhancing TNBC chemo-immunotherapy via combination reprogramming tumor immune microenvironment with immunogenic cell death. Int J Pharm. 2021;598 doi: 10.1016/j.ijpharm.2021.120333. [DOI] [PubMed] [Google Scholar]
- 140.Wang H.R., Tang Y.S., Fang Y.F., Zhang M., Wang H.Y., He Z.D., et al. Reprogramming tumor immune microenvironment (TIME) and metabolism via biomimetic targeting codelivery of Shikonin/JQ1. Nano Lett. 2019;19:2935–2944. doi: 10.1021/acs.nanolett.9b00021. [DOI] [PubMed] [Google Scholar]
- 141.Li J.B., Zhou S., Yu J., Cai W.X., Yang Y.X., Kuang X., et al. Low dose shikonin and anthracyclines coloaded liposomes induce robust immunogenetic cell death for synergistic chemo-immunotherapy. J Control Release. 2021;335:306–319. doi: 10.1016/j.jconrel.2021.05.040. [DOI] [PubMed] [Google Scholar]
- 142.Li J., Zhao M., Xu Y.H., Hu X.Y., Dai Y.H., Wang D.K. Hybrid micelles codelivering shikonin and IDO-1 siRNA enhance immunotherapy by remodeling immunosuppressive tumor microenvironment. Int J Pharm. 2021;597 doi: 10.1016/j.ijpharm.2021.120310. [DOI] [PubMed] [Google Scholar]
- 143.Jiang M., He K.Y., Qiu T., Sun J.H., Liu Q., Zhang X.Q., et al. Tumor-targeted delivery of silibinin and IPI-549 synergistically inhibit breast cancer by remodeling the microenvironment. Int J Pharm. 2020;581 doi: 10.1016/j.ijpharm.2020.119239. [DOI] [PubMed] [Google Scholar]
- 144.Chen Y.N., Hu M.R., Wang S.W., Wang Q., Lu H.Y., Wang F.L., et al. Nano-delivery of salvianolic acid B induces the quiescence of tumor-associated fibroblasts via interfering with TGF-beta1/Smad signaling to facilitate chemo- and immunotherapy in desmoplastic tumor. Int J Pharm. 2022;623 doi: 10.1016/j.ijpharm.2022.121953. [DOI] [PubMed] [Google Scholar]
- 145.Zhang N., Liu S.N., Shi S.Y., Chen Y.T., Xu F.W., Wei X.H., et al. Solubilization and delivery of ursolic-acid for modulating tumor microenvironment and regulatory T cell activities in cancer immunotherapy. J Control Release. 2020;320:168–178. doi: 10.1016/j.jconrel.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 146.Fan L.L., Zhang B.C., Xu A.X., Shen Z.C., Guo Y., Zhao R.R., et al. Carrier-free, pure nanodrug formed by the self-assembly of an anticancer drug for cancer immune therapy. Mol Pharm. 2018;15:2466–2478. doi: 10.1021/acs.molpharmaceut.8b00444. [DOI] [PubMed] [Google Scholar]
- 147.Mao Q.Q., Min J., Zeng R., Liu H.Q., Li H., Zhang C., et al. Self-assembled traditional Chinese nanomedicine modulating tumor immunosuppressive microenvironment for colorectal cancer immunotherapy. Theranostics. 2022;12:6088–6105. doi: 10.7150/thno.72509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghosh B., Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332:127–147. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 149.Li M.Y., Du C.Y., Guo N., Teng Y., Meng X., Sun H., et al. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640–653. doi: 10.1016/j.ejmech.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 150.He H.S., Lu Y., Qi J.P., Zhu Q.G., Chen Z.J., Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9:36–48. doi: 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Y.M., Ye Z.F., Yang H.Y., Xu Q.B. Tailoring combinatorial lipid nanoparticles for intracellular delivery of nucleic acids, proteins, and drugs. Acta Pharm Sin B. 2022;12:2624–2639. doi: 10.1016/j.apsb.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen C., Xia J.X., Ren H.W., Wang A.N., Zhu Y., Zhang R., et al. Effect of the structure of ginsenosides on the in vivo fate of their liposomes. Asian J Pharm Sci. 2022;17:219–229. doi: 10.1016/j.ajps.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barkat M.A., Harshita, Rizwanullah M., Pottoo F.H., Beg S., Akhter S., et al. Therapeutic nanoemulsion: concept to delivery. Curr Pharm Des. 2020;26:1145–1166. doi: 10.2174/1381612826666200317140600. [DOI] [PubMed] [Google Scholar]
- 154.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 155.Lin G., Mi P., Chu C.C., Zhang J., Liu G. Inorganic nanocarriers overcoming multidrug resistance for cancer theranostics. Adv Sci (Weinh) 2016;3 doi: 10.1002/advs.201600134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kus-Liskiewicz M., Fickers P., Ben Tahar I. Biocompatibility and cytotoxicity of gold nanoparticles: recent advances in methodologies and regulations. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mi X.J., Xu X.Y., Choi H.S., Kim H., Cho I.H., Yi T.H., et al. The immune-enhancing properties of Hwanglyeonhaedok-Tang-mediated biosynthesized gold nanoparticles in macrophages and splenocytes. Int J Nanomedicine. 2022;17:477–494. doi: 10.2147/IJN.S338334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang C., Yu H., Yang X.H., Zhang X.B., Wang Y.Q., Gu T.R., et al. Elaborately engineering of a dual-drug co-assembled nanomedicine for boosting immunogenic cell death and enhancing triple negative breast cancer treatment. Asian J Pharm Sci. 2022;17:412–424. doi: 10.1016/j.ajps.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tucker I.M., Burley A., Petkova R.E., Hosking S.L., Penfold J., Thomas R.K., et al. Adsorption and self-assembly properties of the plant based biosurfactant, glycyrrhizic acid. J Colloid Interface Sci. 2021;598:444–454. doi: 10.1016/j.jcis.2021.03.101. [DOI] [PubMed] [Google Scholar]
- 160.Witwer K.W., Wolfram J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 2021;6:103–106. doi: 10.1038/s41578-020-00277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cao M., Yan H.J., Han X., Weng L., Wei Q., Sun X.Y., et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhi K.K., Wang J.C., Zhao H.T., Yang X. Self-assembled small molecule natural product gel for drug delivery: a breakthrough in new application of small molecule natural products. Acta Pharm Sin B. 2020;10:913–927. doi: 10.1016/j.apsb.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang F.H., Su H., Lin R., Chakroun R.W., Monroe M.K., Wang Z.Y., et al. Supramolecular tubustecan hydrogel as chemotherapeutic carrier to improve tumor penetration and local treatment efficacy. ACS Nano. 2020;14:10083–10094. doi: 10.1021/acsnano.0c03286. [DOI] [PubMed] [Google Scholar]
- 164.Wang F.H., Su H., Xu D.Q., Monroe M.K., Anderson C.F., Zhang W.J., et al. Therapeutic supramolecular tubustecan hydrogel combined with checkpoint inhibitor elicits immunity to combat cancer. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121182. [DOI] [PubMed] [Google Scholar]
- 165.Wang F.H., Xu D.Q., Su H., Zhang W.J., Sun X.R., Monroe M.K., et al. Supramolecular prodrug hydrogelator as an immune booster for checkpoint blocker-based immunotherapy. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Younis M.A., Tawfeek H.M., Abdellatif A.A.H., Abdel-Aleem J.A., Harashima H. Clinical translation of nanomedicines: challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181 doi: 10.1016/j.addr.2021.114083. [DOI] [PubMed] [Google Scholar]
- 167.Shan X.T., Gong X., Li J., Wen J.Y., Li Y.P., Zhang Z.W. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12:3028–3048. doi: 10.1016/j.apsb.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bian Z.X., Chen S.L., Cheng C.W., Wang J., Xiao H.T., Qin H.Y. Developing new drugs from annals of Chinese medicine. Acta Pharm Sin B. 2012;2:1–7. [Google Scholar]