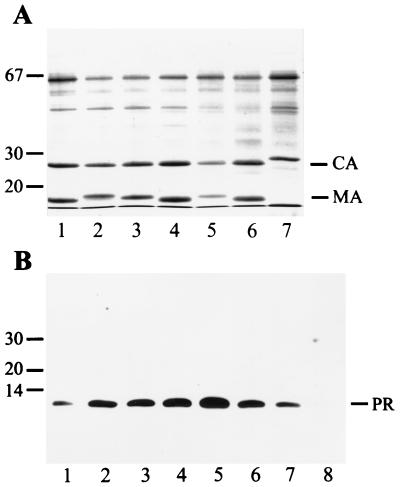
FIG. 1.
Analysis of particle-associated Gag and PR proteins from several HIV-1 and HIV-2 strains. (A) Particles collected by sedimentation through a cushion of sucrose were separated on SDS-polyacrylamide gels, and proteins were detected by Coomassie blue staining. Lanes 1 to 4 show particles from MT4 cells, and lanes 5 to 7 show particles from C8166 cells. HIV-1 strains NL4-3 (lane 1), IIIB (lane 2), HXB2 (lanes 3 and 5), and SF2 (lanes 4 and 6) and HIV-2 strain CBL20 (lane 7) were analyzed in parallel. Molecular mass markers (in kilodaltons) are indicated on the left, and the positions of HIV CA and MA proteins are marked on the right. The 65-kDa protein is bovine serum albumin. (B) Immunoblot detection of particle-associated PR species. Proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell) by electroblotting. Membranes were blocked with 10% low-fat dry milk and reacted with rabbit polyclonal antiserum against HIV-1 PR diluted 1:2,000 in buffer containing 5% low-fat dry milk and 0.01% Tween 20. Incubation was performed overnight at room temperature with shaking. Peroxidase-conjugated antirabbit antiserum (Jackson Immunochemicals Inc.) was used as the secondary antibody at a dilution of 1:10,000 and was incubated for 90 min at room temperature. Immune complexes were visualized with the ECL system (Amersham) according to the manufacturer’s instructions. In lane 1, 50 ng of purified PR was loaded; lanes 2 to 8 contain particle extracts from HIV-1 strains NL4-3 (lane 2), IIIB (lane 3), HXB2 (lanes 4 and 6), and SF2 (lanes 5 and 7) and HIV-2 strain CBL20 (lane 8), as described above. Note that 10 ng of purified PR was analyzed in the same experiment and was detected on a longer exposure.