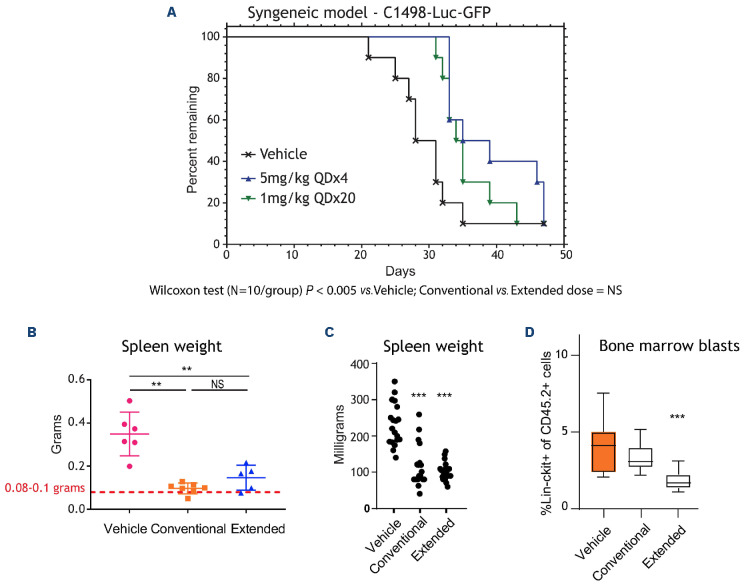
Figure 2.
Extended dosing of Aza preferentially targets bone marrow blasts. (A) Survival of B6 albino mice injected with C1498-Luc3-GFP+ cells and treated with vehicle, conventional-azacitidine (Aza) (5 mg/kg once daily for 4 days [QDx4]) or extended-Aza (1 mg/kg QDx20); N=10 per group. Representative bioluminescence imaging (right panel) of mice are shown (N=1 representative mouse/group). Blue to red color represents low to high intensity of bioluminescence. (B) Mean spleen weights measured at the end of the study for the patient-derived xenograft (PDX) acute myeloid leukemia (AML) mice treated with vehicle, convention-al-Aza (5 mg/kg QDx5) or extended-Aza dosing (1 mg/kg QDx25) are shown as group mean ± standard deviation (N=5 to 7/group). Red dotted line indicates normal C57BL/6J mouse spleen weight. **P ≤ 0.01. (C) Median spleen weights from GEM model of AML harboring FLT3-ITD and TET2 loss (N=17-19/group) (normal spleen weight of wild-type mice is 72 milligrams). *P≤0.05; **P≤0.01, ***P ≤ 0.001 for Aza relative to vehicle/isotype control using non-parametric one-way ANOVA. (D) Flow cytometry was performed on cells collected at the end of week 4 and cell percentages are presented as group medians (N=12/group). ***P≤0.001 for Aza relative to vehicle/isotype control. P values were calculated using non-parametric one-way ANOVA. NS: not significant.