Abstract
We evaluated the efficacy and safety of TAS0313, a multi-epitope long peptide vaccine, plus pembrolizumab in post-chemotherapy immune checkpoint inhibitor—naïve patients with locally advanced/metastatic urothelial carcinoma (la/mUC). TAS0313 9 mg was administered subcutaneously followed by pembrolizumab 200 mg on Day 1, and as monotherapy on Day 8 and 15 of Cycles 1 and 2, and Day 1 of subsequent cycles in 21-day cycles. The primary endpoint was the objective response rate (ORR). Secondary endpoints included progression-free survival (PFS), overall survival (OS), and safety. Biomarkers of response were assessed. In 36 patients enrolled, the ORR was 33.3% (complete response: 7 patients; partial response: 5 patients). Median PFS was 5.0 months; 6- and 12-month progression-free rates were 46.4% and 36.5%, respectively. Median OS was not reached; 6-, 12-, and 24-month OS rates were 83.3%, 72.2%, and 55.1%, respectively. In post hoc analysis, patients with a tumor infiltrating CD8+ lymphocyte (CD8+ TIL) count ≥99 and/or programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥50 and lymphocyte count >1,380 cells/μL had higher ORRs and prolonged PFS versus patients with a CD8+ TIL count <99, PD-L1 CPS <50, and lymphocyte count ≤1,380 cells/μL. Thirty-four (94.4%) patients receiving combination therapy experienced treatment-related adverse events (AE), with pyrexia (n = 15, 41.7%), injection-site reactions (n = 15, 41.7%), injection-site induration (n = 6, 16.7%), and malaise (n = 6, 16.7%) the most common. No grade ≥3 treatment-related AEs occurred in ≥10% of patients. TAS0313 plus pembrolizumab combination therapy showed promising efficacy and manageable safety in la/mUC.
Clinical Trial Registration: JapicCTI-183824.
Introduction
The prognosis for patients with locally advanced or metastatic urothelial carcinoma (la/mUC) is poor, with median time to disease progression of 7.7 months despite first-line platinum-based chemotherapy (1). For mUC, current National Comprehensive Cancer Network guidelines recommend immune checkpoint inhibitor (ICI) monotherapy as second-line therapy, maintenance therapy in the first-line setting, or as first-line therapy in patients with programmed cell death protein 1 (PD-1)-positive disease or those who are ineligible for cisplatin or other platinum-based therapies (2). Although impressive antitumor responses are achieved with anti–PD-1/programmed death-ligand 1 (PD-L1) monotherapy in approximately 17% to 23% of cases (3–7). a significant proportion of patients either fail to respond or lose response over time (8, 9). Novel therapies and/or combinations that enhance the effectiveness of existing therapies are therefore required to improve patient outcomes.
Cancer peptide vaccines are a novel type of cancer immunotherapy that exert their antitumor effect via induction of cytotoxic T lymphocytes (CTL) reactive to known tumor-associated antigens (TAA). To date, a number of clinical studies have been conducted using short peptide vaccines, which have demonstrated partial efficacy in urothelial carcinoma (UC) and glioblastoma (10–12). However, response rates have been low (3%; ref. 13) for several reasons including insufficient immunogenicity of the short peptides (14), CTL dysfunction following accumulation at the antigen-rich injection site, and human leukocyte antigen (HLA) restriction, inducing unfavorable antigen presentation from nonprofessional antigen presenting cells (14).
To overcome the obstacles associated with existing peptide vaccines, an investigational cancer immunotherapeutic vaccine, TAS0313, has been developed that comprises three long peptides (TAS0314, TAS0315, and TAS0316) with a total of 12 CTL epitope peptides restricted by HLA-A24, A2, and A3-supertype (Supplementary Table S1). These peptides are derived from 8 TAAs highly expressed in multiple cancer types, including UC (15). A first-in-human phase I/II clinical study of TAS0313 has recently been conducted in patients with advanced solid tumors. In the phase I dose-finding portion of the study, the safety, tolerability, and induction of immune responses was confirmed (16).
Here, we report the results from Cohort C1 of the aforementioned phase I/II clinical study (16), evaluating the efficacy and safety of TAS0313 in combination with pembrolizumab in patients with la/mUC without prior exposure to ICIs.
Materials and Methods
Study design and treatment
This was a phase I/II open-label, non-randomized, multicenter study to evaluate the safety, tolerability, and efficacy of TAS0313 in patients with solid tumors. The study design consisted of four cohorts: a dose-finding cohort (Cohort A; ref. 16); a efficacy-finding cohort (Cohort B; ref. 17) and two further cohorts, in which the efficacy and safety of TAS0313 and pembrolizumab combination therapy was evaluated in patients with UC without (Cohort C1) and with (Cohort C2) prior exposure to ICIs. Here, we present the results from Cohort C1.
The primary objective was to evaluate the efficacy of TAS0313 and pembrolizumab combination therapy in patients with la/mUC who were naïve to ICIs. A secondary objective was to evaluate the safety of TAS0313 and pembrolizumab combination therapy, which was evaluated in patients with la/mUC without prior exposure to ICIs. As part of the safety assessment, an initial safety evaluation was also conducted in patients with or without prior exposure to pembrolizumab. Exploratory objectives included the assessment of peptide-specific CTL, peptide-specific immunoglobulin G (IgG), and tumor infiltrating CD8+ lymphocyte (CD8+ TIL) counts. mRNA expression levels of target TAA, and protein expression levels of PD-L1 in tumor tissue samples were also assessed.
TAS0313 (Supplementary Table S2) 9 mg was mixed with an adjuvant, Montanide ISA 51 VG and administered subcutaneously near the lymph node on Days 1, 8, and 15 of Cycles 1 and 2, and on Day 1 of subsequent cycles in 21-day cycles (16). Pembrolizumab was administered intravenously following TAS0313 treatment at a dosage of 200 mg on Day 1 of Cycle 1 and each subsequent cycle in 21-day cycles, per the package insert (18). The next cycle had to be started by day 43 at the latest.
The study was conducted in accordance with ethical principles of the Declaration of Helsinki, the International Council for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and the Institutional Review Board (IRB) regulations. All patients or their legally accepted representative provided written informed consent to participate in the study.
Patients
Patients aged ≥20 years were eligible for enrollment if they had: histologically or cytologically confirmed UC of the bladder, renal pelvis, ureter, or urethra that showed predominantly transitional-cell features; ≥1 measurable lesion based on RECIST criteria (19) by imaging; HLA-A*02:01, HLA-A*02:06, HLA-A*02:07, HLA-A*11:01, HLA-A*24:02, HLA-A*31:01, or HLA-A*33:03 allele type; received prior standard therapy including a platinum agent for unresectable la/mUC, or standard pre- or postoperative adjuvant therapy including a platinum-based therapy for localized muscle-invasive UC, with recurrence or progressive disease (PD) confirmed ≤12 months following treatment cessation; received ≤2 prior chemotherapy regimens; an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1; adequate hematologic (absolute neutrophil count of ≥1,500/mm3; hemoglobin value of ≥9.0 g/dL; platelet count of ≥100,000/mm3), renal [serum creatinine ≤1.5 × upper limit of normal (ULN) or creatinine clearance of ≥30 mL/min], and liver (aspartate aminotransferase and alanine aminotransferase ≤3 × ULN, total bilirubin ≤1.5 × ULN) function; a life expectancy of ≥3 months.
Outcome measures
Efficacy
The primary endpoint was the objective response rate [ORR; defined as the proportion of patients achieving complete response (CR) or partial response (PR)], as per RECIST v1.1 (19). Other efficacy endpoints included the disease control rate (DCR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS), as per RECIST v1.1 and immune-related RECIST (iRECIST) criteria (20).
The survival follow-up continued until 18 months after the enrollment of the last subject. On April 22, 2022, all subjects completed follow-up for efficacy endpoints, including OS.
Tumor imaging was conducted at baseline, Week 9, and every 6 weeks thereafter, with assessments conducted by independent central radiologic review.
Immunologic analysis
CTL epitope peptide-specific CTL counts and peptide-specific IgG antibody concentrations were measured from blood samples collected before TAS0313 treatment, and at Day 22 of Cycle 2 and Cycle 3. CTL induction was defined as an increase in CTL count of ≥210 after TAS0313 administration compared with baseline. Human HLA-A*03:01 cDNA and Human HLA-A*24:02:01 cDNA for establishment of antigen presenting cells for CTL detection were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan (21). Detailed methodology regarding the collection and processing of blood and tissue samples for biomarker analysis has been published previously (16). IgG induction was defined as an elevation in IgG of ≥30% to at least one epitope after TAS0313 administration compared with baseline. CD8+ TIL count and protein expression levels of PD-L1 were measured from formalin-fixed, paraffin embedded (FFPE) tumor tissue samples before TAS0313 treatment (mandatory), and at Day 22 of Cycle 2 (optional).
mRNA expression levels of target TAA and HLA-A were measured using nCounter method from FFPE tumor tissue samples before TAS0313 treatment.
Safety
Safety was a secondary endpoint, and was assessed in patients without prior exposure to ICIs as well as the initial safety-evaluable population (see Initial Safety-Evaluable Patients’ Section for additional details), which included patients without prior exposure to ICIs and with prior exposure to pembrolizumab.
Safety assessments in patients without prior exposure to ICIs included the incidence of adverse events (AE), including serious AEs, treatment-related AEs, and laboratory variables. Adverse events were evaluated and categorized by system organ class (SOC) and preferred term using the Medical Dictionary for Regulatory Activities version 23.1, and their severity graded according to the Common Terminology Criteria for Adverse Events, version 4.03.
Data were also collected on vital signs, body weight, laboratory variables, and 12-lead electrocardiogram findings.
Initial Safety-Evaluable Patients:
The first 6 patients, or 12 patients (if an additional 6 patients were enrolled) who received TAS0313 and pembrolizumab combination treatment, irrespective of whether they were ICI-naïve or whether they had prior exposure to pembrolizumab, were evaluated for treatment-related AEs that met the safety evaluation criteria.
If 2 of 6 patients experienced a treatment-related AE that met the initial safety evaluation criteria below, 6 patients were enrolled. If ≥3 of 6 patients, or ≥4 of 12 patients, experienced a treatment-related AE that met the initial safety evaluation criteria, the study was discontinued.
Initial Safety Evaluation Criteria:
The safety evaluation criteria for hematologic toxicity included: (i) any Grade 4 hematologic toxicity; (ii) Grade 3 thrombocytopenia associated with clinically significant bleeding; (iii) febrile neutropenia (absolute neutrophil count <1,000/mm3 with a single body temperature of >38.3°C or sustained fever of ≥38°C for >1 hour); (iv) anemia requiring blood transfusion; or (v) thrombocytopenia requiring blood transfusion.
Safety evaluation criteria for nonhematologic toxicity were: (i) any Grade 4 nonhematologic toxicity; (ii) any Grade 3−4 nonhematologic toxicity (except Grade 3 fatigue lasting ≤3 days; Grade 3 diarrhea, nausea, or vomiting in the absence of symptomatic treatment with anti-emetics or anti-diarrheals; Grade 3 rash in the absence of symptomatic treatment with corticosteroids or anti-inflammatory drugs; (iii) Grade 3−4 nonhematologic laboratory value if clinically significant medical intervention is required, or the abnormality leads to hospitalization, persists for >7 days, or results in a drug-induced liver injury; (iv) inability to start treatment within 14 days from the scheduled Day 1 of Cycle 2, or ≥2 doses are skipped, due to treatment-related toxicity; (v) any Grade 5 toxicity.
Statistical analysis
Efficacy endpoints were evaluated using the full analysis set (FAS), which comprised all enrolled patients who met the eligibility criteria and received ≥1 dose of study drug. With the exception of the initial safety evaluation, safety analyses were performed using the safety analysis set, which comprised all patients who received ≥1 dose of study drug. The pharmacodynamic-evaluable population included all patients who received ≥1 dose of study drug and had relevant data (e.g., CTL, IgG, CD8+ TIL) available. The pharmacogenomic-evaluable population included all patients who received ≥1 dose of study drug and had relevant data (e.g., mRNA expression of target TAA and HLA-A, the analysis of HLA-A typing and protein expression of PD-L1) available.
In terms of planned enrollment, a maximum of 60 patients was planned to be registered prior to study initiation. However, following an amendment to the statistical analysis plan during the study, a total of 36 patients were analyzed. At a threshold response rate of 15%, an expected response rate of 35% and a one-sided significance level of 5%, the power was 86% for 36 patients. The threshold response rate was determined on the basis of the lower limit of the 95% confidence interval (CI) in the phase III KEYNOTE−045 study (3). The null hypothesis was rejected and TAS0313 was judged to be effective when more than 10 of 36 patients responded.
Baseline demographics were summarized using descriptive statistics, with median (min−max) calculated for continuous variables, and the frequency number and proportion calculated for categorical variables.
Time-to-event analyses (PFS, 6- and 12-month progression-free rate, and 6-, 12-, and 24-month OS rates) were summarized using the Kaplan–Meier method with 95% CI, HR and two-sided 95% CI based on a Cox model and P values were calculated by log rank test. P values of ORR and DCR were calculated by Fisher exact test.
ROC were constructed as a post hoc analysis to evaluate the effect of CD8+ TIL and/or PD-L1 combined positive score (CPS), and lymphocyte count, on ORR and DCR, with PR and CR represented as “positive” and SD and PD represented as “negative” responses.
The CTL cut-off value for our study was defined as +3 standard deviations above the pre-treatment mean for the negative control samples across all enzyme‐linked immunospot (ELISPOT) assays.
The frequency of AEs was summarized descriptively overall and for each individual event (by SOC and preferred term).
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Patient disposition and baseline characteristics
A total of 36 patients were enrolled between May 7, 2019 and May 26, 2020. All (100.0%) patients met the eligibility criteria and received ≥1 dose of study drug and comprised the FAS and safety analysis set, respectively. Among the FAS, 36 (100.0%) patients discontinued treatment with TAS0313. The primary reason for treatment discontinuation was PD (n = 20, 55.6%). Other reasons included next cycle treatment delays of more than 21 days (n = 5, 13.9%), AEs (n = 3, 8.3%), completion of ≥35 treatments of pembrolizumab (n = 4, 11.1%), investigator decision (n = 3, 8.3%), and recurrent Grade 2 pneumonitis (n = 1, 2.8%). Baseline demographic and clinical characteristics of patients in the FAS are presented in Table 1.
Table 1.
Demographic and baseline clinical characteristics.
| FAS | |
|---|---|
| (N = 36) | |
| Age, years, median (min, max) | 71.0 (52.0, 84.0) |
| Age, years, category | |
| <65 | 10 (27.8) |
| ≥65 | 26 (72.2) |
| Sex | |
| Male | 31 (86.1) |
| ECOG PS | |
| 0 | 28 (77.8) |
| 1 | 8 (22.2) |
| Smoking status | |
| Current | 2 (5.6) |
| Former | 27 (75.0) |
| Never | 7 (19.4) |
| Histologic type | |
| Pure transitional cell | 25 (69.4) |
| Predominantly transitional cell | 11 (30.6) |
| Cancer type | |
| Bladder | 16 (44.4) |
| Renal pelvis | 14 (38.9) |
| Ureter | 6 (16.7) |
| Bellmunt risk factor scorea | |
| 0 | 13 (36.1) |
| 1 | 14 (38.9) |
| 2 | 3 (8.3) |
| ≥3 | 6 (16.7) |
| History of surgery | 32 (88.9) |
| Previous platinum therapy | |
| Cisplatin | 23 (63.9) |
| Carboplatin | 12 (33.3) |
| Nedaplatin | 1 (2.8) |
| Previous systemic drug therapies | |
| Neoadjuvant | 7 (19.4) |
| Adjuvant | 12 (33.3) |
| Advanced/metastatic | 25 (69.4) |
| Maintenance | 1 (2.8) |
| Intravesical infusion | 5 (13.9) |
| Other | 0 |
| HLA-A Type | |
| HLA-A*02:01 | 7 (19.4) |
| HLA-A*02:06 | 5 (13.9) |
| HLA-A*02:07 | 3 (8.3) |
| HLA-A*11:01 | 4 (11.1) |
| HLA-A*24:02 | 24 (66.7) |
| HLA-A*31:01 | 10 (27.8) |
| HLA-A*33:03 | 4 (11.1) |
| Other | 9 (25.0) |
| Tumor PD-L1 CPS, 10% cutoff | |
| <10% | 18 (51.4) |
| ≥10% | 17 (48.6) |
Note: Data presented as n (%) unless otherwise specified.
Abbreviations: CPS, combined positive score; HLA, human leukocyte antigen; ECOG PS, Eastern Cooperative Oncology Group Performance Status.
aBellmunt risk factor is defined according to the number of following criteria that are met: 1) ECOG PS > 0; 2) Hemoglobin <10 g/dL at baseline; 3) Presence of liver metastasis; 4) Time from prior chemotherapy <3 months.
Treatment
The median [interquartile range (IQR)] number of treatment cycles was 7.5 (4.0−11.5), the median (IQR) treatment duration was 161.0 (106.0−284.5) days, and the median (IQR) total administered dose of TAS0313 and pembrolizumab was 103.5 (72.0−139.5) mg and 1,500.0 (800.0−2,200.0) mg, respectively.
Efficacy
Clinical responses with TAS0313 treatment are summarized in Supplementary Table S3. The ORR (95% CI) in the FAS was 33.3% (18.6−51.0), including CR in 7 (19.4%) patients and PR in 5 (13.9%) patients, according to RECIST. As the lower limit of the CI exceeds the threshold response rate (15%), the null hypothesis was rejected at a one-sided significance level of 0.05, and the primary endpoint of the study was met. Stable disease was confirmed in a further 12 (33.3%) patients according to RECIST, and the DCR (95% CI) was 66.7% (49.0–81.4). No difference in response rates were observed when analyzed according to iRECIST criteria. On the basis of the results of the post hoc ROC analyses, a cut-off value of ≥99 for CD8+ TILs (Supplementary Fig. S1A) and ≥50 for PD-L1 CPS (Supplementary Fig. S1B) as well as a cut-off value of >1,380 cells/μL for lymphocyte count (Supplementary Fig. S1C) were identified as predictive markers for response.
A scatter plot showing the distribution of response by baseline CD8+ TIL and PD-L1 CPS category is presented in Fig. 1. The ORR was 57.1% (95% CI, 28.9−82.3) in patients with a baseline CD8+ TIL count ≥99 and/or PD-L1 CPS ≥50, and 19.0% (95% CI, 5.4−41.9) in patients with a baseline CD8+ TIL count <99 and PD-L1 CPS <50 (P = 0.031). The DCR was 78.6% (95% CI, 49.2−95.3) in patients with a baseline CD8+ TIL count ≥99 and/or PD-L1 CPS ≥50, and 61.9% (95% CI, 38.4−81.9) in patients with a baseline CD8+ TIL count <99 and PD-L1 CPS <50 (P = 0.461; Supplementary Table S3). The ORR was 53.8% (95% CI, 25.1−80.8) in patients with a lymphocyte count >1,380 cells/μL, and 21.7% (95% CI, 7.5−43.7) in patients with a lymphocyte count ≤1,380 cells/μL (P = 0.071). The DCR was 84.6% (95% CI, 54.6−98.1) in patients with a lymphocyte count >1,380 cells/μL, and 56.5% (95% CI, 34.5−76.8) in patients with a lymphocyte count ≤1,380 cells/μL, (P = 0.143; Supplementary Table S3). There was no difference in response by lesion site.
Figure 1.
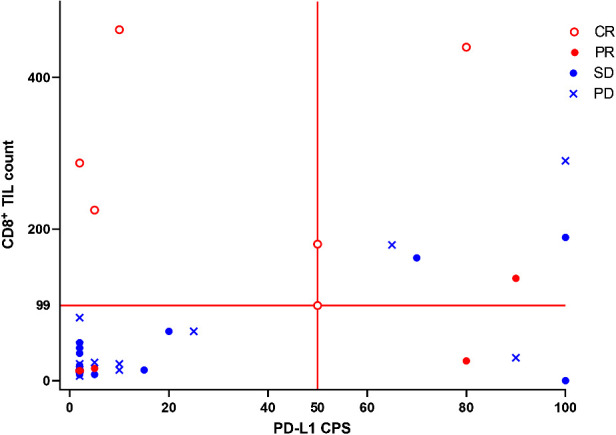
Scatter plot of best overall response with TAS0313 by baseline tumor-infiltrating CD8+ lymphocyte and PD-L1 CPS.
Kaplan–Meier estimates of PFS in the FAS and by baseline PD-L1 CPS and lymphocyte count are presented in Fig. 2. Median PFS (95% CI) was 5.0 (2.4–14.2) months. The median PFS was 26.4 months (95% CI, 2.3 months–NE) in patients with a baseline CD8+ TIL count ≥99 and/or PD-L1 CPS ≥50, and 3.7 months (95% CI, 2.1–10.4 months) in patients with a baseline CD8+ TIL count <99 and PD-L1 CPS <50, (HR, 0.350; 95% CI, 0.135–0.910; P = 0.024). The median PFS was 18.7 months (95% CI, 3.7 months–NE) in patients with a lymphocyte count >1,380 cells/μL, and 3.5 months (95% CI, 2.1–10.4 months) in patients with a lymphocyte count ≤1,380 cells/μL, (HR, 0.427; 95% CI, 0.174–1.048; P = 0.055; Fig. 2B and C).
Figure 2.
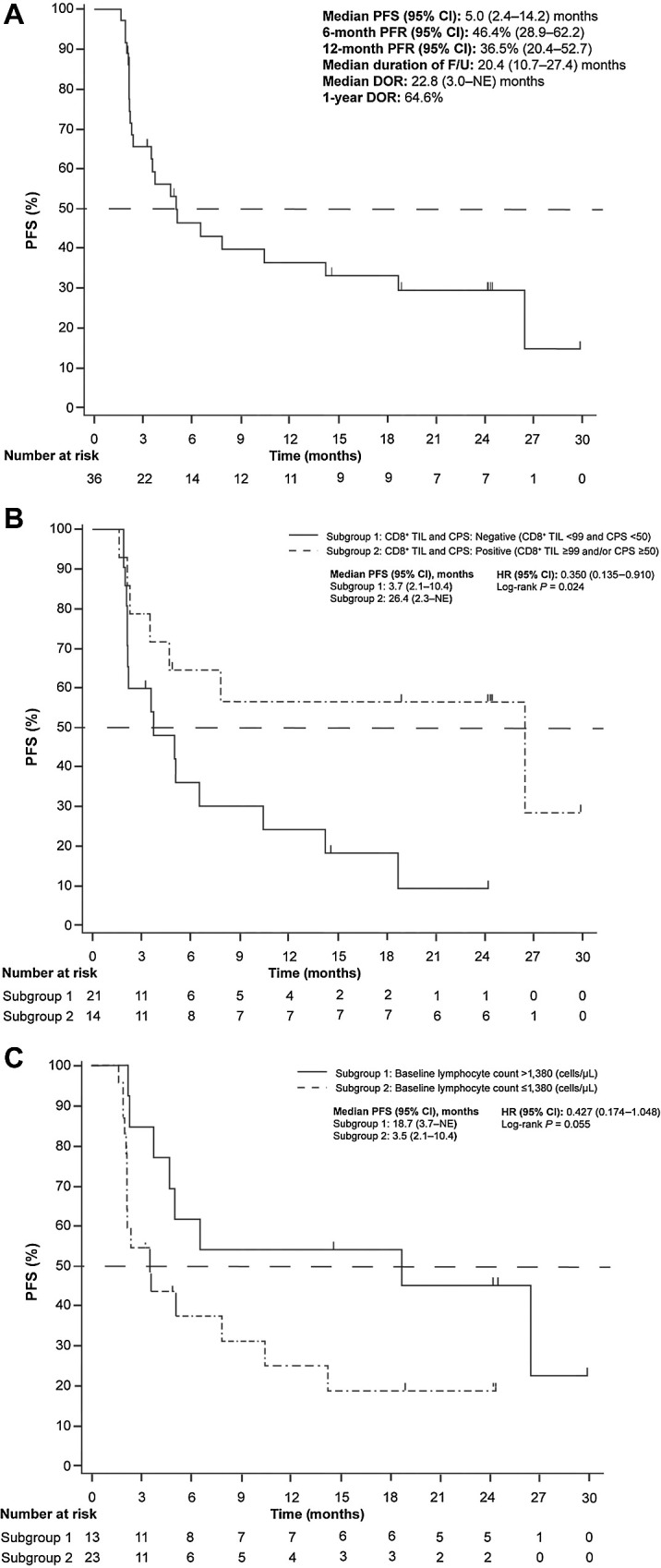
Kaplan–Meier estimate of PFS (A) in the FAS and by (B) baseline tumor-infiltrating CD8+ lymphocyte level CPS and (C) lymphocyte count - (Independent Central Radiological Review)
Median OS (95% CI) in the FAS was not reached, and 72.2% (54.5−84.0) of patients were alive at 12 months (Fig. 3A). The median OS was not reached (95% CI, 10.7 months–NE) in patients with a baseline CD8+ TIL count ≥99 and/or PD-L1 CPS ≥50, and 18.8 months (95% CI, 10.8 months–NE) in patients with a baseline CD8+ TIL count <99 and PD-L1 CPS <50, (HR, 0.304; 95% CI, 0.086–1.072; P = 0.050). The median OS was not reached (95% CI, 10.5 months–NE) in patients with a lymphocyte count >1,380 cells/μL, and 20.2 months (95% CI, 10.8 months–NE) in patients with a lymphocyte count ≤1,380 cells/μL, (HR, 0.635; 95% CI, 0.223–1.808; P = 0.391; Fig. 3B and C).
Figure 3.
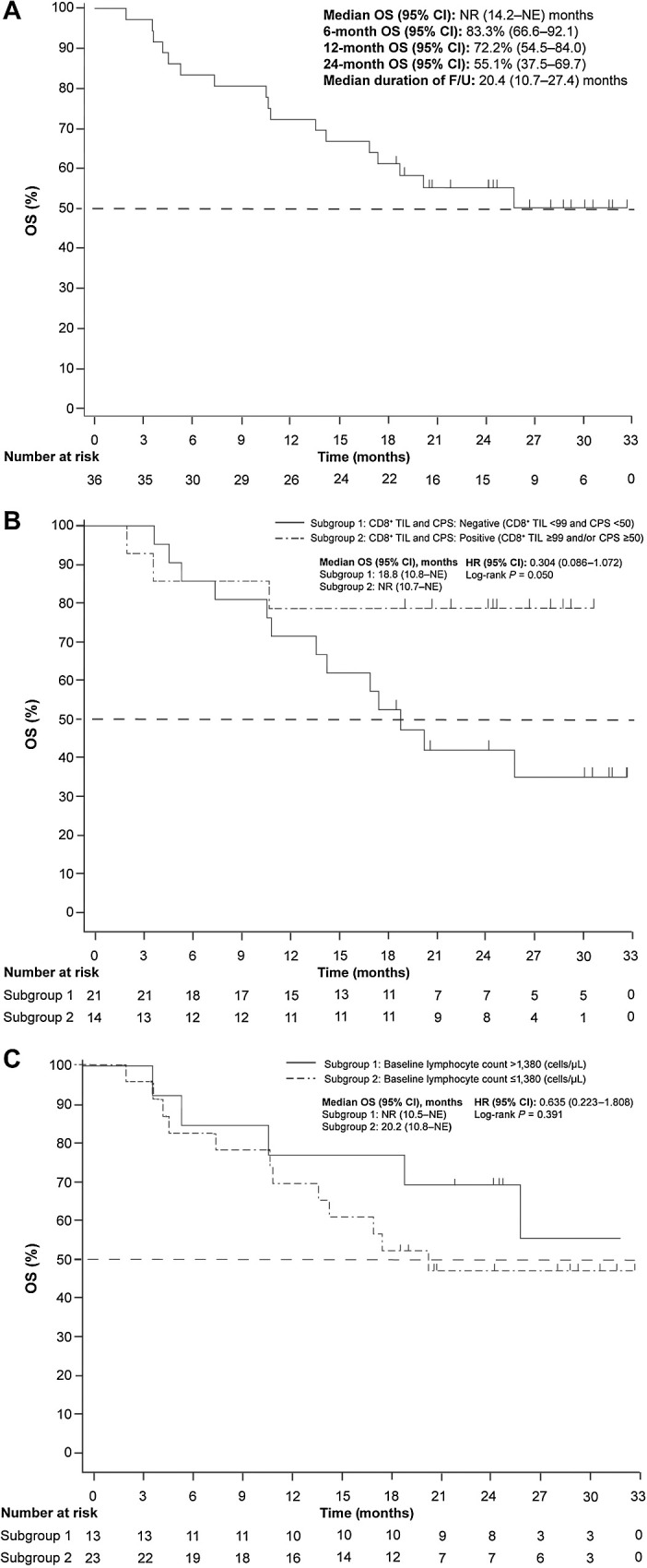
Kaplan–Meier estimate of OS (A) in the FAS and by (B) baseline tumor-infiltrating CD8+ lymphocyte level CPS and (C) lymphocyte count - (Independent Central Radiological Review)
Peptide-specific CTL and IgG induction occurred in 15/34 (44.1%) patients and 35/35 (100.0%) patients respectively, following TAS0313 administration, confirming the immune activity of TAS0313. No clear correlation between peptide-specific immune response and efficacy was observed.
Safety
Tolerability and safety of TAS0313 and pembrolizumab combination treatment was confirmed in 6 patients (who received prior pembrolizumab treatment) enrolled in the initial safety evaluable population. Of these, 1 patient developed a treatment-related AE (respiratory failure) that met the prespecified initial safety evaluation criteria.
The incidence of AEs and treatment-related AEs occurring during TAS0313 and pembrolizumab combination treatment are presented in Supplementary Table S4; Supplementary Table S5, respectively. A total of 34 (94.4%) patients experienced treatment-related AEs during the study, 14 (38.9%) of which were grade ≥3 in severity. The most common (≥10%) treatment-related AEs by preferred term were pyrexia and injection site reactions [n = 15 each (41.7%)] followed by injection site induration and malaise [n = 6 each (16.7%)]. Dermatologic injection site reactions (n = 25, 69.4%) were a notable AE, consisting of injection site reaction (n = 15, 41.7%), injection site induration (n = 6, 16.7%), injection site pruritus (n = 4, 11.1%), injection site pain (n = 3, 8.3%), injection site ulcer (n = 3, 8.3%), injection site abscess (n = 1, 2.8%), injection site erythema (n = 1, 2.8%), injection site infection (n = 1, 2.8%), and injection site swelling (n = 1, 2.8%). No grade 4 treatment-related AEs occurred. The only grade 3 treatment-related AE that occurred in >1 patient was adrenal insufficiency [n = 2 (5.6%)]. No dose interruptions occurred due to AEs. Three (8.3%) patients discontinued treatment-related AEs [Grade 3 febrile neutropenia (n = 1); Grade 3 rash (n = 1); recurrent Grade 2 pneumonitis (n = 1)]. Three (8.3%) patients failed to start the next cycle by Day 43 of each cycle because physicians determined TAS0313 and pembrolizumab administration needed to be delayed due to treatment-related AEs outside of the administration start criteria [Grade 1 pneumonitis (n = 1); Grade 2 myositis (n = 1), Grade 2 injection site ulcer (n = 1)].
Tummor-associated antigens and HLA-A expression
Of the 36 patients, expression of the eight target TAAs and HLA-A were confirmed in all enrolled patients (Supplementary Table S1 and S2).
Discussion
In this phase I/II open-label study, we report the results of the first study evaluating the combination of a long-peptide vaccine and an anti–PD-1 antibody in patients with ICI-naïve la/mUC. The null hypothesis was set to be rejected when the lower limit of the CI exceeded the threshold response rate (15%), and the TAS0313 and pembrolizumab combination was deemed effective in this population. An ORR of 33.3% was achieved in our study, with 12 responses, including 7 cases of CR. The median PFS was 5.0 months. The median OS (95% CI) was not reached, and 72.2% (54.5−84.0) of patients were alive at 12 months. The ORR, the CR rate, and median PFS and OS in our study were greater than that reported with pembrolizumab monotherapy in the KEYNOTE-45 study (21.1%, 7.0%, 2.1 months, and 10.3 months, respectively; ref. 3), and also tended to be greater than that achieved with other ICI monotherapies (approximately 13%–21%, 2%−6%, 2 months, and 7−18 months, respectively; refs. 4–7, 22). Surprisingly, the clinical outcome of this trial appears to be comparable with the results achieved with a recently reported highly immunogenic personalized neoepitope peptide vaccine plus pembrolizumab trial in a bladder cancer cohort (22). The DOR in the pembrolizumab single-agent KEYNOTE-045 study was 29.7 months (23), while the DOR in our study was 22.8 months, showing a trend toward shorter DOR. The difference in follow-up period (20.4 months for the median follow-up and 62.9 months for the median follow-up of KEYNOTE-045) should be taken into consideration, but further study is needed. Nevertheless, our results demonstrate the efficacy and safety of TAS0313 in combination with pembrolizumab and suggest that TAS0313 may have an advantage as a highly effective off-the-shelf cancer peptide vaccine-based therapy.
Expression of target TAAs and HLA-A was observed in all patients, confirming the immune activity of TAS0313. Furthermore, TAS0313 treatment was effective across multiple HLA phenotypes, including patients with HLA-A02, -A03, -A11, -A24, -A31, and -A33 and no correlation was observed between HLA phenotype and efficacy, suggesting that TAS0313 is likely to be effective for a wide range of patients with solid tumors. Importantly, the most common treatment-related AEs were dermatologic injection site reactions (n = 25, 69.4%) and pyrexia (n = 15, 41.7%) and there did not appear to be an increase in the incidence of treatment-related AEs compared with pembrolizumab alone despite the potent antitumor effect observed (3, 18). No major safety concerns occurred with the TAS0313 and pembrolizumab combination
Of the 34 (94.4%) patients who had CTL levels evaluated before treatment, 15 (44.1%) experienced CTL induction following TAS0313 treatment. Although satisfactory, the proportion of patients experiencing an increase in CTL counts was lower than that reported with other cancer peptide vaccines (24, 25). Reasons for this discrepancy may be partially explained by differences in the assay method used between studies. To overcome blood sample limitations and accommodate the numerous types of peptides, we opted to employ a culture of peripheral blood mononuclear cells (PBMC) expanded for 6 days with a peptide cocktail, distinguishing our approach from conventional studies that solely used a single peptide without expanding PBMCs. Further, it cannot be ruled out that the presence of pembrolizumab may have interfered with the sensitivity of epitope peptides (comprising TAS0313)-specific CTL detection, because ICI will be able to activate both cancer-associated antigen specific and non-specific T cells.
In terms of the prognostic biomarker analysis, patients with a high baseline CD8+ TIL count, PD-L1 CPS, or lymphocyte count at baseline experienced superior responses and prolonged PFS compared with patients with a low baseline CD8+ TIL, PD-L1 CPS, or lymphocyte count, consistent with the results of the phase I dose-finding cohort (Cohort A) of TAS0313 monotherapy and below reports (16). In the KEYNOTE-045 study (3), the PFS was similarly prolonged among patients with a PD-L1 CPS ≥10% at baseline compared with a PD-L1 CPS <10%. In another study of patients treated with pembrolizumab monotherapy for UC, a higher median CD8+ TIL at baseline was associated with longer cancer-specific survival (HR, 0.068; 95% CI, 0.01−0.57) and DOR compared with low median CD8+ TIL at baseline (26). The results of this study were indicating that a high CD8+ TIL count and/or PD-L1 CPS, and lymphocyte count at baseline may serve as useful biomarkers of response to combination therapy. In addition, OS tended to be prolonged in patients with high baseline CD8+ TIL counts and PD-L1 CPS. Conversely, there was no significant difference in OS between high and low baseline lymphocyte counts. This may be due to the protocol criteria for discontinuation of OS follow-up, in which many patients were censored and a longer follow-up period was necessary to obtain accurate data.
The additive and synergistic effects of therapeutic cancer peptide vaccines and ICIs have been demonstrated in a number of preclinical and clinical studies (27–30). Specifically, our previous preclinical study demonstrated that the combination therapy using TAS0314 and PD-1 blockade enhanced the number of tumor infiltrating TAS0314-induced epitope-specific CTLs compared with each monotherapy (27). On the basis of the preclinical data and previous clinical trial results, TAS0313 may increase the efficacy of ICIs favoring the transition of immunologically cold tumors into immunologically hot tumors. In turn, ICIs may increase the efficacy of the TAS0313 vaccine via inhibition of immunosuppressive regulatory signaling associated with immune system evasion, thereby increasing the magnitude of vaccine-mediated T-cell responses.
Because we could not perform serial biopsy in this study, we cannot discuss the relationship between changes in CD8+ TIL and PD-L1 CPS expression and drug efficacy. However, when the tumor microenvironment was in a favorable state for CTL infiltration and/or PD-L1 CPS before drug treatment, TAS0313-induced CTL also infiltrated into tumors (16, 27), potentially providing a greater clinical benefit to the patients. More detailed immunologic studies are needed to explain the high combined efficacy of TAS0313 and PD-1 blockade in future clinical trials.
Other limitations of the study included the relatively small sample size (n = 36), and potential lack of generalizability of the findings due to differences in HLA type between Japanese and other races (31). Further, patient background factors (such as CPS) are different compared with previous studies (3). An independent control arm was also not included due to the preliminary nature of the study, making comparisons difficult. The promising efficacy and manageable safety profile observed in this study support the use of TAS0313 in combination with pembrolizumab, although confirmation in a larger, randomized controlled study is warranted.
Ethics approval and consent to participate
The clinical study protocol, investigator's brochure, sample informed consent form, and other study-related documents were reviewed and approved by the IRBs of all study sites. Each investigator conducted the study according to applicable local or regional regulatory requirements and in accordance with the ethical principles of the Declaration of Helsinki, the International Council for Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice, and IRB regulations.
All patients or their legally accepted representative provided written informed consent to participate in the study.
Data availability
The results of Cohort C2 have been previously reported at the American Society of Clinical Oncology 2021 annual meeting.
This manuscript is intended to submit the results of Cohort C1. As the initial safety evaluation could not be confirmed in C1, initial safety data from Cohort C2 has also been included, with a statement that the initial safety evaluation in patients with urothelial cancer was conducted in a separate cohort.
Data will be available upon request.
Supplementary Material
Supplementary Legends
Table S1, Table S2, Table S3, Table S4, Table S5
Figure S1
Acknowledgments
The authors thank all clinicians for their involvement and contribution to the study. Medical writing assistance was provided by Jordana Campbell, BSc, CMPP of inScience Communications, Springer Healthcare. This medical writing assistance was funded by Taiho Pharmaceutical Co., Ltd.
This study was funded by Taiho Pharmaceutical Co., Ltd. Funding was provided by Taiho Pharmaceutical Co., Ltd to author institutions for study/manuscript support.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Authors' Disclosures
H. Nishiyama reports personal fees from Taiho Pharmaceutical Co., Ltd. during the conduct of the study; personal fees from Merck Sharp & Dohme (MSD), AstraZeneca, Nippon Kayaku Co. Ltd., Merck Biopharma Co. Ltd., Janssen; grants and personal fees from Astellas, Ono Pharmaceutical; and grants from Chugai Pharma outside the submitted work. N. Matsubara reports personal fees from Sanofi; grants and personal fees from Janssen; grants from AstraZeneca, Bayer, Roche, MSD, Taiho, Astellas, Amgen, Eisai, Eli Lilly, PRA Health Science, Takeda, Pfizer, Seagen, Chigai; and grants from AbbVie outside the submitted work. M. Eto reports grants from Sanofi, Ono Pharmaceutical, Takeda Pharmaceutical; personal fees from MSD, Ono Pharmaceutical, Takeda Pharmaceutical, Merck, AstraZeneca, Eisai, Bristol-Myers Squibb, Astellas Pharma, Pfizer; and personal fees from Janssen Pharmaceutical outside the submitted work. W. Obara reports other support from Taiho Pharmaceutical Co., Ltd. during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
H. Nishiyama: Investigation, methodology, writing–original draft, writing–review and editing. J. Yonese: Investigation, writing–original draft, writing–review and editing. T. Kawahara: Investigation, writing–original draft, writing–review and editing. R. Matsumoto: Investigation, writing–original draft, writing–review and editing. H. Miyake: Investigation, writing–original draft, writing–review and editing. N. Matsubara: Investigation, writing–original draft, writing–review and editing. H. Uemura: Investigation, writing–original draft, writing–review and editing. M. Eto: Investigation, writing–original draft, writing–review and editing. H. Azuma: Investigation, writing–original draft, writing–review and editing. W. Obara: Investigation, writing–original draft, writing–review and editing. A. Terai: Investigation, writing–original draft, writing–review and editing. S. Fukasawa: Investigation, writing–original draft, writing–review and editing. S. Suekane: Investigation, writing–original draft, writing–review and editing.
References
- 1. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8. [DOI] [PubMed] [Google Scholar]
- 2. NCCN guidelines for bladder cancer V2021. Available from: https://www.nccn.org. Date Accessed: 10 Sept 21.
- 3. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. New Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicenter, open-label, phase III randomized controlled trial. Lancet 2018;391:748–57. [DOI] [PubMed] [Google Scholar]
- 5. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicenter, single-arm, phase II trial. Lancet Oncol 2017;18:312–22. [DOI] [PubMed] [Google Scholar]
- 6. Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase I trial. Lancet Oncol 2018;19:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase I/II open-label study. JAMA Oncol 2017;3:e172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah S, Wood K, Labadie B, Won B, Brisson R, Karrison T, et al. Clinical and molecular features of innate and acquired resistance to anti–PD-1/PD-L1 therapy in lung cancer. Oncotarget 2018;9:4375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh RJ, Soo RA. Resistance to immune checkpoint inhibitors in non–small cell lung cancer: biomarkers and therapeutic strategies. Therapeutic Adv Med Oncol 2020;12:1758835920937902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noguchi M, Matsumoto K, Uemura H, Arai G, Eto M, Naito S, et al. An open-label, randomized phase II trial of personalized peptide vaccination in patients with bladder cancer that progressed after platinum-based chemotherapy. Clin Cancer Res 2016;22:54–60. [DOI] [PubMed] [Google Scholar]
- 11. Terasaki M, Shibui S, Narita Y, Fujimaki T, Aoki T, Kajiwara K, et al. Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen–A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2011;29:337–44. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto N, Tsuboi A, Kagawa N, Chiba Y, Izumoto S, Kinoshita M, et al. Wilms tumor 1 peptide vaccination combined with temozolomide against newly diagnosed glioblastoma: safety and impact on immunological response. Cancer Immunol Immunother 2015;64:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Med 2004;10:909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melief CJM, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nature Rev Cancer 2008;8:351–60. [DOI] [PubMed] [Google Scholar]
- 15. Noguchi M, Koga N, Moriya F, Suekane S, Yutani S, Yamada A, et al. Survival analysis of multiple peptide vaccination for the selection of correlated peptides in urological cancers. Cancer Sci 2018;109:2660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondo S, Shimizu T, Koyama T, Sato J, Iwasa S, Yonemori K, et al. First-in-human study of the cancer peptide vaccine TAS0313 in patients with advanced solid tumors. Cancer Sci 2021;112:1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narita Y, Okita Y, Arakawa Y. Evaluation of the efficacy and safety of TAS0313 in adults with recurrent glioblastoma. Cancer Immunol Immunother 2022;71:2703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keytruda [prescribing information]. Merck & Co., Inc, 2021. Available from: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdfDate Accessed: 30 Apr 2021. [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 20. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akatsuka Y, Goldberg TA, Kondo E, Martin EG, Obata Y, Morishima Y, et al. Efficient cloning and expression of HLA class I cDNA in human B-lymphoblastoid cell lines. Tissue Antigens 2002;59:502–11. [DOI] [PubMed] [Google Scholar]
- 22. Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, et al. A Phase Ib trial of personalized neoantigen therapy plus anti–PD-1 in patients with advanced melanoma, non–small cell lung cancer, or bladder cancer. Cell 2020;183:347–62. [DOI] [PubMed] [Google Scholar]
- 23. Balar AV, Castellano DE, Grivas P, Vaughn DJ, Powles T, Vuky J, et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol 2023;34:289–99. [DOI] [PubMed] [Google Scholar]
- 24. Iwasa S, Yamada Y, Heike Y, Shoji H, Honma Y, Komatsu N, et al. Phase I study of a new cancer vaccine of ten mixed peptides for advanced cancer patients. Cancer Sci 2016;107:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noguchi M, Arai G, Matsumoto K, Naito S, Moriya F, Suekane S, et al. Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration-resistant prostate cancer: dose-related immune boosting and suppression. Cancer Immunol Immunother 2015;64:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng B, Park JH, Ren L, Yew PY, Kiyotani K, Antic T, et al. CD8 lymphocytes in tumors and nonsynonymous mutational load correlate with prognosis of bladder cancer patients treated with immune checkpoint inhibitors. Cancer Rep 2018;1:e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka Y, Wada H, Goto R, Osada T, Yamamura K, Fukaya S, et al. TAS0314, a novel multi-epitope long peptide vaccine, showed synergistic antitumor immunity with PD-1/PD-L1 blockade in HLA-A*2402 mice. Sci Rep 2020;10:17284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dutoit V, Marinari E, Dietrich P-Y, Migliorini D. CTIM-08. Combination of the IMA950/Poly-ICLC multipeptide vaccine with pembrolizumab in relapsing glioblastoma patients. Neuro Oncol 2020;22:ii34. [Google Scholar]
- 29. Dey S, Sutanto-Ward E, Kopp KL, DuHadaway J, Mondal A, Ghaban D, et al. Peptide vaccination directed against IDO1-expressing immune cells elicits CD8+ and CD4+ T-cell–mediated antitumor immunity and enhanced anti–PD-1 responses. J ImmunoTher Cancer 2020;8:e000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwala SS, O'Day SJ, Zakharia Y, Voorhies B, Milhem M. 1378TiP - A phase I clinical trial investigating the therapeutic cancer vaccine UV1 in combination with pembrolizumab as first-line treatment of patients with malignant melanoma. Ann Oncol 2019;30:v562. [Google Scholar]
- 31. Middleton D, Williams F, Meenagh A, Daar AS, Gorodezky C, Hammond M, et al. Analysis of the distribution of HLA-A alleles in populations from five continents. Human Immunol 2000;61:1048–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Legends
Table S1, Table S2, Table S3, Table S4, Table S5
Figure S1
Data Availability Statement
The results of Cohort C2 have been previously reported at the American Society of Clinical Oncology 2021 annual meeting.
This manuscript is intended to submit the results of Cohort C1. As the initial safety evaluation could not be confirmed in C1, initial safety data from Cohort C2 has also been included, with a statement that the initial safety evaluation in patients with urothelial cancer was conducted in a separate cohort.
Data will be available upon request.


