Abstract
Human malaria is known to be transmitted strictly by anopheline mosquitoes. Culicine mosquitoes such as Aedes spp. and Culex spp. are important vectors of other human pathogens including viruses and filarial worms, but have never been observed to transmit mammalian malarias. Culicines do transmit avian malarias, and interestingly, allow partial development of mammalian-infectious Plasmodium parasites, implying that physiological barriers in the mosquitoes prevent parasite transmission. Although the mechanism(s) are not known, the mosquito immune system is likely involved in eliminating Plasmodium. However, Plasmodium has shown substantial capacity to adapt to new vectors, and current ecological changes caused by humans could promote adaptation of human-infectious Plasmodium parasites to culicines. Such an event could have widespread epidemiological implications and therefore merits attention.
Keywords: malaria, transmission, mosquito, Culex, Anopheles
Mosquitoes that transmit human Plasmodium spp
Malaria is a devastating disease, which, despite all control efforts, still causes ~2 million clinical cases and 660 000 deaths annually [1]. The disease is caused by Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, Plasmodium knowlesi, and most importantly by Plasmodium falciparum, which is responsible for the greatest burden in terms of morbidity and mortality. Human-infectious Plasmodium spp., in fact all Plasmodium spp. that infect mammals, are known to be transmitted via the bite of an infected anopheline mosquito (see Glossary). However, culicine mosquitoes (e.g., Aedes spp., Culex spp., and Mansonia spp.), which are common worldwide, are also abundantly present in areas where malaria is endemic. They feed on humans and are, just like anophelines, important vectors for human pathogens, transmitting filarial worms and arboviruses (e.g., Yellow fever, Dengue, and West Nile Virus). Additionally, culicine mosquitoes are the vectors for avian Plasmodium spp. (Table 1). Nevertheless, they do not transmit human (mammalian) malaria parasites, and what prevents transmission remains a mystery. Besides having significant epidemiological implications, solving this question might shed light onto Plasmodium biology and its host specificity and help in uncovering powerful mechanisms that block Plasmodium development in the mosquito.
Table 1.
Examples of pathogens transmitted by anopheline and culicine mosquitoes in nature and the laboratory
| Pathogen | Mosquito | |||||
|---|---|---|---|---|---|---|
| Anophelinae | Culicinae | Refs | ||||
| Anopheles spp. | Culex spp. | Aedes spp. | Mansonia spp. | |||
| Human malaria | P. falciparum | ✓a | Ookinetesc [2], SGsporozoitesd [6] | N/E | Oocystsg [11] | [51, 52] |
| P. vivax | ✓ | SGsporozoites [6] | N/E | N/E | ||
| P. malariae | ✓ | SGsporozoites [6] | N/E | N/E | ||
| P. ovale | ✓ | N/E | N/E | N/E | ||
| P. knowlesi | ✓ | N/E | Sporozoitesf (mentioned in [5], [53] | N/E | ||
| Simian malaria | P. cynomolgi | ✓ | N/E | N/E | Sporozoites [5] | [54–56] |
| P. reichenowi | ✓ | N/E | N/E | |||
| Rodent malaria | P. berghei | ✓ | No exflagellatione [4] | No exflagellation [3] | N/E | [57] |
| P. yoelii | ✓ | N/E | N/E | N/E | ||
| P. chabaudi | ✓ | N/E | N/E | N/E | ||
| Avian malaria | P. gallinaceum | ✓ | ✓ | ✓ | N/E | [3, 58] |
| P. relictum | ✓ | ✓ | ✓ | N/E | ||
| Flavi-viruses | Dengue | N/Eb | N/E | ✓ | N/E | [59–62] |
| Yellow fever | N/E | N/E | ✓ | N/E | ||
| West Nile | N/E | ✓ | ✓ | ✓ | ||
| Japanese encephalitis | ✓ | ✓ | ✓ | N/E | ||
| St. Louis encephalitis | ✓ | ✓ | ✓ | N/E | ||
| α-viruses | O’nyong’nyong | ✓ | N/E | N/E | N/E | [63–66] |
| Sindbis | N/E | ✓ | N/E | N/E | ||
| Chikungunya | ✓ | ✓ | ✓ | ✓ | ||
| Lymphatic filariasis | Wuchereria bancrofti | ✓ | ✓ | ✓ | ✓ | [67] |
| Brugia malayi | ✓ | ✓ | ✓ | ✓ | ||
✓: successful transmission has been observed in nature and/or in the laboratory.
N/E: no evidence.
Ookinetes: ookinetes have been found in the blood meal and on the midgut epithelium of the mosquito.
SGsporozoites: sporozoites can reach the salivary glands (SG) of the mosquito.
No exflagellation: activation of male gametocytes is impaired, and no exflagellation can be observed.
Sporozoites: sporozoites are present in the mosquito hemolymph but do not invade the salivary glands.
Oocysts: oocysts were observed on the midgut epithelium.
For the adaptation of a parasite to a new vector in nature, several conditions have to be met in terms of vector competence and capacity (Box 1). This would require at least one parasite genotype overcoming all of the physiological barriers encountered in the new vector, and ecological circumstances promoting adaptation to a new vector, which would necessitate a higher benefit than cost to the parasite.
Box 1. Determinants of vectorial capacity and competence.
The ability of a mosquito to transmit a pathogen depends on environmental, behavioral, cellular, and biochemical factors that encompass the vectorial capacity of the mosquito. Among these factors are host preference, feeding rate and behavior, mosquito population density, longevity, and its susceptibility to transmit the pathogen, also known as vectorial competence. A number of environmental (reviewed in [68]) and physiological parameters can determine the vectorial competence to Plasmodium, including:
Components of the mammalian host complement system are ingested during a blood meal by the mosquito vector and can be deleterious to Plasmodium in the midgut of the mosquito. In response to this, P. falciparum gametes have developed the ability to inactivate complement protein C3b by co-opting the complement regulating factor H from the blood [69].
Plasmodium male gametogenesis is triggered by mosquito-released xanthurenic acid (XA) [13].
The mosquito gut harbors microbiota that increase substantially during blood digestion and can block Plasmodium development by triggering a mosquito immune response or by inhibiting the parasite directly through the production of reactive oxygen species [70].
The chitinous peritrophic matrix forms during digestion and lines the luminal side of the midgut epithelium, representing a physical barrier that the parasite must cross. Some Plasmodium parasites secrete chitinases to disrupt the peritrophic matrix [71].
The mosquito innate immune system can develop an immune response that in some cases eliminates Plasmodium. The midgut epithelium is the first mosquito tissue that the parasite crosses. Inside the epithelial cell, production of reactive oxygen species [72] and protein nitration mediated by a peroxidase (HPX2) and a NADPH-oxidase (NOX5) system can lead to Plasmodium elimination [73]. Protein nitration of Plasmodium appears to be a prerequisite for recognition and elimination of the parasites by the mosquito complement-like immune system. This involves the binding of thioester-containing protein 1 (TEP1) to the parasite to label it for destruction. Recently it has been found that P. falciparum parasites are able to evade the complement-like system and recognition by TEP1 in An. gambiae by a mechanism involving Pfs47, a parasite surface protein [74].
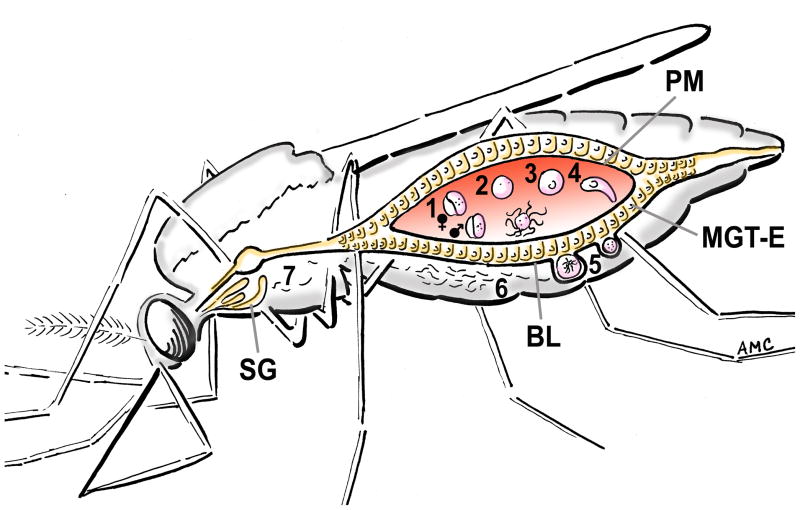
During the “normal” life cycle of Plasmodium within a susceptible mosquito (Figure 1), male and female gametocytes are taken up with the blood while the mosquito feeds on an infected vertebrate host (Figure 1, step 1). Inside the midgut lumen of the mosquito, the gametocytes are “activated” and transform into gametes (Figure 1, step 2), male gametes (microgametes) exflagellate and fertilize the females (macrogametes), and within an hour a diploid zygote is formed (Figure 1, step 3). The zygote develops into a motile ookinete (Figure 1, step 4) that crosses the mosquito midgut epithelium after 16 to 26 hours and transforms into an oocyst on the basal side of the midgut (Figure 1, step 5). Sporozoites mature in the oocyst and are released into the hemolymph after approximately 10–16 days (Figure 1, step 6). The sporozoites travel to the salivary glands (Figure 1, step 7), from where they are transmitted to another vertebrate host during a subsequent blood feeding of the mosquito. Culicine mosquitoes undoubtedly take up human-infectious Plasmodium gametocytes; however, parasites are not transmitted. Several questions arise concerning why this is the case: (i) what are the barriers that human malaria parasites encounter in culicines; (ii) can any of the human-infectious Plasmodium spp. overcome these barriers and be transmitted by a culicine mosquito; and (iii) what ecological conditions would promote the adaptation of human-infectious Plasmodium spp. to culicines?
Figure 1. Plasmodium development in a mosquito vector.
Successful transmission of Plasmodium spp. by a mosquito involves a complex developmental cycle within the vector. The mosquito must: (1) ingest a blood meal containing male and female Plasmodium gametocytes; (2) within minutes the gametocytes develop into gametes; (3) female macrogametes are fertilized by the male microgametes and a diploid zygote is formed; (4) the zygote develops into a motile ookinete; (4) the ookinete crosses the peritrophic matrix (PM) to invade the midgut epithelium (MGT-E) by 16-26 h post-feeding. (5) Successful ookinetes traverse the midgut epithelial cells and form oocysts, lying between the basal membrane of the epithelium and the basal lamina (BL). (6) The oocyst takes 7–21 days to develop thousands of sporozoites that are released into the mosquito hemolymph. (7) A fraction of the sporozoites invade the salivary glands (SG) and remain there to be injected into another vertebrate host when the mosquito takes another blood meal. Each step in the life cycle of Plasmodium in the mosquito can potentially represent a barrier for transmission (Box 1).
Here, we discuss these questions in the context of the few studies that have investigated the development of mammalian Plasmodium spp. in culicine mosquitoes, including recent findings showing that the human malaria parasite P. falciparum can develop ookinetes in Culex quinquefasciatus and infects its midgut epithelium [2], indicating that a human-infectious malaria parasite is already able to develop partially in a culicine mosquito.
The development of mammalian Plasmodium spp. in culicine mosquitoes
Several studies have tested if different human, primate, and other mammalian malaria parasites can complete their development in culicine mosquitoes. Depending on the mosquito and Plasmodium species used, parasites were observed in more or less advanced stages of development, ranging from the absence of ookinetes in the midgut lumen [3, 4] to the presence of sporozoites in the salivary glands of the mosquito (Figure 1, steps 4–7) [5, 6]. Only a few studies have investigated development of human-infectious Plasmodium spp. in culicine mosquitoes. The earliest study of P. falciparum in a culicine mosquito of which we are aware was performed in 1937 by Williamson and Zain, who infected Culex bitaeniorhyncus with P. falciparum and other human-infectious Plasmodium spp. They observed oocysts on the midgut epithelium of the mosquitoes and sporozoites in the salivary glands after infections with P. falciparum, P. vivax, and P. malariae [6]. This observation is the closest to completion of the developmental cycle of human malaria parasites in a culicine mosquito found thus far. However, in another study with C. bitaeniorhyncus and P. falciparum, no oocysts were observed on the midgut epithelium [7]. It remains unclear if the results were not replicated because of the use of different genotypes of parasite and vector [8–10].
In infections of Mansonia uniformis with P. falciparum, oocysts were observed on the midgut epithelium after feeding on an infected patient. Unfortunately, no mosquitoes were kept long enough to see if sporozoites would develop in the oocysts and invade the salivary glands [11]. A recent study of the development of P. falciparum in C. quinquefasciatus showed that the parasites develop into ookinetes in the blood meal of this mosquito in nearly the same numbers as in their natural vector, Anopheles gambiae [2]. But immediately after ookinetes cross the midgut epithelium and reach the basal side, they are lysed and no oocysts are seen.
Parasite development to near completion has not only been observed for human-infectious Plasmodium spp. [6], but also for the primate malaria parasite Plasmodium cynomolgi. In infections of M. uniformis, oocysts as well as sporozoites were found in the mosquitoes [5]. Yet, the sporozoites were seen in the hemolymph of the mosquitoes, but seemed to fail to invade the salivary glands. Only one sporozoite was reported to be inside a gland, and transmission experiments were unsuccessful, confirming that no infectious sporozoites were present in the salivary glands of the mosquitoes.
Conversely, the most extreme arrest of development was described for the rodent malaria parasite Plasmodium berghei. In studies with Aedes aegypti [3] and Culex salinarius [4], only limited ookinete formation could be observed in the blood meal of the mosquitoes, leading to the conclusion that the ookinetes were unable to cross the midgut epithelium [4]. This assumption was confirmed later; analyses of P. berghei in the blood meals of Ae. aegypti revealed that the transformation of male gametocytes into microgametes (measured as the number of exflagellations observed) was highly affected and rarely seen [3]. Feeding experiments with in vitro cultured P. berghei ookinetes, carried out in the same study, showed that significantly fewer ookinetes attach to the midgut epithelium in Ae. aegypti than in Anopheles stephensi. No oocysts were observed 48 h post-feeding, suggesting that P. berghei ookinetes are unable to cross the Ae. aegypti midgut epithelium [3]. Even when injected directly into the mosquito hemolymph, the ookinetes did not form oocysts, and sporozoites were never observed in the salivary glands of Ae. aegypti compared to An. stephensi, where 93% of the mosquitoes had high numbers of sporozoites in their glands 18–20 days after parasite injection. As would be expected, transmission experiments with P. berghei using Ae. aegypti were unsuccessful [3].
Taken together, the above described observations from Plasmodium spp. that can infect mammals point to several conclusions: first, there is no evidence for complete development and successful transmission of any of the studied parasites by a culicine mosquito. Second, the ability of mammalian-infectious Plasmodium parasites to develop in culicine mosquitoes is highly dependent on the combination of mosquito and parasite species used. It is apparent that each of the studied parasite species is affected at a different developmental stage in the mosquito (Figure 1) and therefore seems to encounter a unique physiological barrier (Box 1). This suggests that several mechanisms are responsible for the developmental arrest/death of the parasites and that species-specific adaptation is required for successful completion of parasite development in a vector.
A number of factors are known from studies of Plasmodium in Anopheles mosquitoes that are either required by the parasite or controlling infection. For example, it has been shown that P. berghei parasites need xanthurenic acid (XA), a metabolite found in the mosquito, to initiate exflagellation of male gametes in the midgut [12]. In Ae. aegypti and C. salinarius, P. berghei development is arrested at the early stage of microgamete activation, immediately after parasites reach the mosquito midgut (Figure 1, step 1) [3, 4]. XA concentrations are lower in culicine mosquitoes than in anophelines, and the sensitivity of the parasites to XA varies between Plasmodium spp. [12, 13]. Because P. berghei requires relatively high concentrations of XA, this could explain the lack of exflagellating microgametes in Ae. aegypti. This could be tested directly by increasing the concentration of XA in the blood meal by providing it to the mosquito or injecting it into a P. berghei infected mouse before mosquito infection. In addition to the components that are promoting parasite development, the mosquito blood meal contains factors such as vertebrate complement, antibodies, and mosquito gut microbiota, all of which have been shown to be harmful for the parasite (Box 1) and could be responsible for a developmental arrest. In the case of P. falciparum, the fact that parasites can be found on the basal side of the midgut epithelium [2], or in some cases even form sporozoites in culicine mosquitoes [5, 6], indicates that P. falciparum, in contrast to P. berghei, is able to cope with the conditions in the midgut lumen, cross the peritrophic matrix, and invade the midgut epithelial cells. In C. quinquefasciatus, the parasites are lysed immediately after they reach the basal side of the midgut epithelium and come into contact with the mosquito hemolymph. In insects, the hemolymph contains factors of the innate immune system, and members of several different immune pathways have been identified as major defenses against malaria parasites in Anopheles mosquitoes. The most powerful anti-plasmodial response known to date is probably achieved by the thioester-containing protein TEP1, a homologue of the human complement factor C3 [14–16]. TEP1 is a hemolymph protein released by hemocytes. It forms a complex with two leucine-rich repeat (LRR) proteins, LRIM1 (leucine-rich repeat immune protein 1) and APL1 (Anopheles-Plasmodium-responsive leucine-rich repeat 1), preventing it from non-specific binding [17–19]. Binding of TEP1 to Plasmodium ookinetes or early oocysts initiates their elimination and further lysis or melanization in An. gambiae [14] and has been shown to be the major determinant of refractoriness of Anopheles quadriannulatus [20]. Additionally, several other major immune pathways (IMD-, TOLL, JAK-STAT, JNK-pathways) have been shown to influence infection intensities in both P. berghei and P. falciparum infections [21–24]. Analyses of the C. quinquefasciatus and Ae. aegypti genomes (www.vectorbase.org) and the Immune gene database ImmunoDB (http://cegg.unige.ch/Insecta/immunodb) reveal the presence of several thioester-containing proteins, which could be part of a complement-like system in these culicine mosquitoes, as well as components of the IMD-, TOLL-, and JNK pathways. RNA-mediated silencing of these genes (which should lead to the survival of the parasites), could give insights into the role of the mosquito immune system in parasite killing in culicines. Silencing of some TEP genes in C. quinquefasciatus show an effect in immunity against bacteria (Knöckel, J., unpublished), indicating that they do have a function in culicine immunity, but their effect on Plasmodium has to be determined.
Other explanations for the observed death of P. falciparum in C. quinquefasciatus could be a lack of specific nutrients or signals that are needed for the parasites to continue their development. However, in other culicine mosquitoes, sporozoites have been observed, some of which were located in the salivary glands of the mosquitoes. Plasmodium ookinetes can also transform into oocysts when injected into the Drosophila hemolymph [25], suggesting that lack of nutrients or chemical signals is an unlikely barrier. Mammalian-infectious Plasmodium spp. have been successful in adapting to anopheline mosquitoes throughout the world, but have not (yet) been shown to complete their development in culicine mosquitoes. Nonetheless, the question that comes to mind following the observations that mammalian Plasmodium can at least partially develop in culicine mosquitoes is: is there any Plasmodium sp. that has the capacity to be transmitted by both anopheline and culicine mosquitoes?
Transmission of Plasmodium spp. by anopheline and culicine mosquitoes
Avian malaria parasites are typically transmitted by culicine mosquitoes in nature (Table 1). Nevertheless, under laboratory conditions, Plasmodium gallinaceum has been successfully transmitted by Anopheles quadrimaculatus [26–28], An. stephensi, and An. gambiae [3, 29, 30]. Oocysts were observed on the midgut epithelium of Anopheles freeborni, but transmission of the parasites by the mosquitoes was not tested [26]. In the case of Plasmodium relictum, transmission between birds by anopheline mosquitoes has been demonstrated with An. quadrimaculatus, Anopheles albimanus [28, 31], Anopheles crucians [28], and An. freeborni [28, 32].
Even though many parasites seem to be eliminated in the midgut epithelium [33], a recent study showed that An. stephensi can transmit P. gallinaceum to chickens [30]. Feeding on an infected chicken led to infection in 2% of the mosquitoes. Furthermore, a highly susceptible An. stephensi line was genetically selected, which showed over 85% infection prevalence with P. gallinaceum within a few generations. A similar approach was used 25 years earlier by Collins and coworkers, who selected a Plasmodium-refractory and a Plasmodium-susceptible line of An. gambiae [29]. The selection was based on the observation that a small fraction of the colonized mosquitoes showed reduced susceptibility to P. cynomolgi, a primate malaria parasite. In those refractory mosquitoes, large numbers of melanized parasites could be observed on the midgut epithelium alongside a few or no live oocysts. Selective breeding using these mosquitoes, or on the other hand the mosquitoes that showed the highest level of live oocysts after infection, led to the establishment of two mosquito lines, one fully refractory and one highly susceptible to P. cynomolgi. Interestingly, the selection not only conferred refractoriness or susceptibility to P. cynomolgi, but also to the avian parasite P. gallinaceum and other Plasmodium spp., including some P. falciparum lines [29]. All parasite species tested are mostly encapsulated by melanization in the refractory An. gambiae. Since encapsulation is a major immune response to fight invading pathogens, this suggests that the mosquito immune system is responsible for the observed phenotypes. More recently, it has been confirmed that the complement-like immune system is mediating the selected refractoriness in the case of P. falciparum [34]. This example clearly shows that the mosquito immune system is an important barrier for Plasmodium to adapt to a new vector.
Ecological aspects of pathogen adaptation to a new vector
In addition to the physiological barriers that the parasite encounters inside the mosquito, ecological factors must be in favor of promoting transmission. The benefit of adapting to a new vector has to be higher than any cost associated with a decrease in transmission efficiency in the original vector. Under certain ecological circumstances, pathogens are able to rapidly adapt to new vectors. One example is the Chikungunya virus (CHIKV), originally transmitted by Ae. aegypti. A single point mutation resulting in an amino acid replacement significantly increased the transmission efficiency of the virus in Aedes albopictus [35]. This same point mutation has occurred independently in at least three different geographical areas in just a few years, resulting in new disease epicenters: the islands of the Indian Ocean (e.g., La Reunion), Central Africa, and Asia [35–37]. During these adaptations Ae. albopictus was common in all three areas, whereas the original vector, Ae. aegypti was rare [35, 37]. Notably, the new mutant form of CHIKV spread in Ae. albopictus in La Reunion despite causing elevated mortality of the new vector [36]. Recently, it was shown that the mutation in CHIKV is also associated with slightly lower transmission efficiency by Ae. aegypti [38]. These studies suggest that pathogen adaptation to new vectors is governed to a large extent by the cost/benefit ratio, which can include reduced transmission efficiency in the original vector. Reducing access of the parasite to its original vector increases the prospects for an adaptation to a new, more abundant vector species. In the case of Plasmodium, similar ecological conditions may have occurred during the arrival of the parasites in the New World. It resulted in its adaptation to the local anopheline species, which had diverged from their African counterparts ~95 million years ago [39], when the continents separated. One example is P. vivax adaptation to different anophelines in Mexico [40]. In coastal areas, where An. albimanus is most common, the parasites are transmitted by An. albimanus, whereas at higher altitudes the main vector is An. pseudopunctipennis, a “foothill-mosquito” [41]. The populations of P. vivax in these geographic areas are genetically distinct, and consistent with a local adaptation of a parasite to a vector, the coastal An. albimanus is more susceptible to infection by the coastal P. vivax genotype, whereas An. pseudopunctipennis is more susceptible to foothill parasite genotypes [40].
The establishment of the parasite in new lands almost always depends on vector switches [42, 43]. Nevertheless, human-infecting Plasmodium spp. have only adapted to different anopheline species, but not to culicine mosquitoes. However, an adaptation to culicines changes in a way that promotes it. Although not specifically designed for this purpose (and maybe not reaching the tipping point to shift adaptive prospects), a significant change of ecological conditions has been occurring for several decades in Africa due to urbanization [44, 45] and large scale vector control in rural areas [45]. Because anophelines cannot tolerate pollution in urban centers whereas culicines can, vector composition in cities is dominated by culicines [44, 46–49]. Likewise, successful vector control campaigns in many rural areas, especially in East Africa, resulted in dramatic suppression of anopheline vectors with far less impact on C. quinquefasciatus [50]. The cost-benefit ratios for malaria parasites in Africa may be changing as these lines are written. The prevailing trends increase the benefit of adaptation to a new vector such as C. quinquefasciatus.
An ideal experiment to test the ability of human-infectious malaria parasites to adapt to culicine mosquitoes could involve exposure of diverse parasite populations to large numbers of mosquitoes that represent “poor” vector species and testing parasite transmission to the vertebrate host over time in a series of replicated experiments. But which parasite-vector combinations would provide us with the most valuable information to predict the likelihood of a vector switch to happen in nature? Certainly, highly abundant mosquitoes with frequent human host contact that lasts long enough to allow sporozoite development and transmission would represent promising candidates for adaptation to a new vector. Nonetheless, since adaptation initially depends on rather low but successful transmission by the new vector, a mosquito that allows current parasites to develop into sporozoites (such as M. uniformis and P. cynomolgi) appears to be a better candidate than a mosquito that does not allow parasite development (P. berghei in Ae. aegypti). Accordingly, even if P. falciparum can complete development in C. bitaeniorhyncus (see above), it is not known to bite people in Africa, and therefore it represents a poor candidate for parasite adaptation in Africa. Nevertheless, it is common in Asia where it feeds on people frequently and thus warrants further attention. However, its wide distribution and variable phenotype suggest that it represents a species complex (http://www.wrbu.org/SpeciesPages_non-ANO/non-ANO_A-hab/CXbit_hab.html).
Concluding remarks
We discussed here why human (mammalian)-infectious Plasmodium spp. are not transmitted by culicine mosquitoes. Based on the fact that culicines such as Aedes spp. and Culex spp. are the main vectors for other human pathogens and are present in areas where malaria is endemic, their contact with human malaria parasites in nature is indisputable. It has been demonstrated that several mammalian-infectious Plasmodium spp. can reach advanced developmental stages in some culicine mosquitoes; nevertheless, there is no evidence that culicines transmit malaria to humans. This suggests that the physiological barriers in the mosquito are either too strong in culicines and/or the cost of adaptation to a new vector is still too high for the parasite. It has been repeatedly shown that the mosquito immune system plays a crucial role in defining vector competence of Anopheles mosquitoes for Plasmodium spp., and manipulating the mosquito immune response can influence parasite survival. Present evidence suggests that the culicine immune system is involved in preventing transmission of mammalian-infectious Plasmodium spp. On the other hand, Plasmodium has shown great capacity to adapt to new vectors on different continents, and recent results show that P. falciparum parasites are able to evade the mosquito immune system in An. gambiae. The observation that avian Plasmodium spp. can be transmitted by anopheline and culicine mosquitoes clearly indicates that the same could be possible for mammalian-infectious Plasmodium parasites. Based on our current knowledge it is difficult to say if this is ever going to happen and when, and more research would be needed to provide a more reliable prediction (Box 2). The current decrease in Anopheles population density in African urban areas concurrent with an increase in culicine mosquitoes could move the balance toward adaptation of the parasite to culicines. Such adaptation could have significant consequences for public health and therefore attention for this possibility may be prudent.
Box 2. Outstanding questions.
What is the nature of the physiological barriers that prevent transmission of mammalian-infectious Plasmodium spp. by culicines?
Which culicine species are more likely to transmit mammalian malaria?
What are the precise ecological conditions that would favor adaptation of human-infectious Plasmodium spp. to culicine mosquitoes?
How important is the parasite’s fitness cost tradeoff when adapting to a different vector?
Can we be sure that transmission of human malaria by culicine mosquitoes has not happened in nature?
Highlights.
Culicine mosquitoes are exposed to human-infectious Plasmodium spp, but do not transmit it.
Culicines allow partial development of several mammalian Plasmodium spp.
Different Plasmodium spp. encounter diverse barriers that prevent transmission.
The right ecological conditions might promote adaptation for transmission.
Acknowledgments
We would like to thank Louis H. Miller and Jose Ribeiro (Laboratory for Malaria and Vector Research, NIAID, NIH), Scott Weaver (University of Texas Medical Branch, Galveston, Texas), Peter Armbruster (Georgetown University, Washington DC), and Louis Lambrechts (Institut Pasteur, Paris, France) for helpful suggestions and insightful comments on the manuscript and Jose Luis Ramirez for assistance in image processing.
Glossary
- Anopheline mosquito
mosquitoes are classified within the Culicidae family (“small flies”), which is, based on morphology, divided into two subfamilies, the Anophelinae and the Culicinae
- Basal lamina (BL)
layer of extracellular matrix secreted by epithelial cells on the basal side of the epithelium, serving as support for the epithelial cells. Two major components of the BL are the proteins laminin and collagen
- Complement factor C3b
plasma protein derived from Factor C3 that binds to antigens on pathogens to label them for destruction
- Complement-like immune system
a part of the insect immune system, analogous to the vertebrate complement system. It includes the thioester-containing protein 1 (TEP1) and leucine-rich repeat proteins LRIM1 and APL1
- Culicine mosquito
see anopheline mosquito
- Exflagellation
process by which Plasmodium male gametes are released from the red blood cell. It is identified under the microscope as male gametes move their flagella vigorously
- Factor H
serum protein component of the vertebrate complement system that inhibits complement activity by binding complement protein C3b
- Gamete
sexually differentiated cell that fuses with a gamete of different sex to form a fertilized zygote during sexual reproduction. Plasmodium gametes are formed in the mosquito midgut lumen and are derived from gametocytes ingested by a mosquito in a blood meal
- Gametocyte
sexual stages of Plasmodium, which are formed in the peripheral blood of an infected vertebrate host. They exist in two forms, male and female gametocytes
- Hemolymph
circulatory fluid in insects similar to blood in vertebrates
- IMD-pathway
immune signaling pathway that is primarily activated by gram- bacteria and has been shown to respond against P. falciparum in An. gambiae
- JNK-pathway
the Jun-N-terminal kinase (JNK)-pathway appears to be involved in responding to oxidative stress, and regulates cell growth, differentiation, survival and apoptosis (cell death) and immunity. The pathway mediates an immune response against the rodent malaria parasite P. berghei in An. gambiae mosquitoes
- Melanization
part of the innate immune response of insects and other arthropods in the defense against pathogens. Pathogens are coated by a layer of the black melanin pigment and killed. Melanization is also a mechanism of wound healing, covering injuries with melanin
- Midgut epithelium (MGT-E)
single layer of cells lining the mosquito gut. The cells are lying on a basal lamina that separates them from the hemolymph and contain microvilli on the anterior side facing the midgut lumen. The main function of the midgut epithelium is secretion of digestive enzymes and absorption of nutrients
- Midgut lumen
interior cavity of the mosquito midgut where the blood meal is contained and digested
- Oocyst
Plasmodium developmental stage formed from ookinetes after they crossed the midgut epithelial cells. The oocysts are located on the basal side of the midgut epithelium between the basal membrane of the epithelial cell and the basal lamina and give rise to thousands of sporozoites
- Ookinete
motile stage of the Plasmodium parasite that develops in the midgut lumen of the mosquito from the fertilized zygote and crosses the midgut epithelium to come to rest on the basal side of the midgut cell, where it develops into an oocyst
- Peritrophic matrix (PM)
chitin-containing membrane that is formed in the mosquito midgut after blood feeding. Matrix components are secreted by the midgut epithelial cells and surround the blood bolus, protecting the midgut epithelium from gut microbiota and pathogens
- Salivary glands (SG)
saliva-producing glands of the mosquito. Plasmodium sporozoites invade the salivary glands in order to be transmitted to another vertebrate host in the mosquito saliva
- Sporozoite
infectious stage of Plasmodium. Sporozoites are formed in the oocysts and are released approximately 10 days after the mosquito blood meal. They invade the salivary glands and are transmitted to a vertebrate host during a blood meal of the mosquito. In the vertebrate host, sporozoites infect the liver and give rise to merozoites that infect the red blood cells
- Thioester-containing protein 1 (TEP1)
protein found in the hemolymph of mosquitoes, which has structural similarity to complement factor C3. Upon cleavage, it binds to the surface of Plasmodium and tags it for elimination
- TOLL-pathway
Signaling pathway involved in insect embryonic development and immunity. It is mainly activated by gram+ bacteria, fungi, and viruses and has been shown to act against the rodent malaria parasite P. berghei in An. gambiae
- Vectorial capacity
ability of a mosquito to transmit a pathogen (see Box 1)
- Vectorial competence
susceptibility of a mosquito to be infected by a pathogen (see Box 1)
- Vertebrate complement
part of the immune system of vertebrates, composed of serum proteins including “Factor C3” that upon activation lead to tagging of a pathogen for destruction
- Xanthurenic acid (XA)
byproduct of eye-pigment synthesis in insects
- Zygote
diploid cell, product of the fusion of two haploid gametes during sexual reproduction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Malaria Report 2012. 2012. [Google Scholar]
- 2.Knockel J, et al. An impossible journey? The development of Plasmodium falciparum NF54 in Culex quinquefasciatus. PLoS One. 2013;8:e63387. doi: 10.1371/journal.pone.0063387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alavi Y, et al. The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int J Parasitol. 2003;33:933–943. doi: 10.1016/s0020-7519(03)00112-7. [DOI] [PubMed] [Google Scholar]
- 4.Yoeli M. Plasmodium berghei: Mechanisms and sites of resistance to sporogonic development in different mosquitoes. Exp Parasitol. 1973;34:448–458. doi: 10.1016/0014-4894(73)90104-5. [DOI] [PubMed] [Google Scholar]
- 5.Warren M, et al. Primate malaria infection in Mansonia uniformis. Mosq News. 1962;22:303–304. [Google Scholar]
- 6.Williamson KB, Zain M. A presumptive culicine host of the human malaria parasites. Trans R Soc Trop Med Hyg. 1937:XXXI. [Google Scholar]
- 7.Singh J, Mohan BN. Susceptibility of Culex (culex) bitaeniorhynchus Giles, 1901, to Plasmodium relictum but not to Plasmodium gallinaceum and Plasmodium falciparum. Indian J Malariol. 1955;9:71–74. [PubMed] [Google Scholar]
- 8.Lambrechts L, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol. 2009;9:160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrechts L, et al. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Malar J. 2005;4:3. doi: 10.1186/1475-2875-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrechts L, et al. Can transgenic mosquitoes afford the fitness cost? Trends Parasitol. 2008;24:4–7. doi: 10.1016/j.pt.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Cheong WH, et al. The experimental infection of Plasmodium falciparum in a culicine mosquito Mansonia uniformis. Singapore Med J. 1963;4:183–184. [Google Scholar]
- 12.Billker O, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 13.Arai M, et al. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol Biochem Parasit. 2001;116:17–24. doi: 10.1016/s0166-6851(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 14.Blandin S, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 15.Blandin SA, et al. Antimalarial responses in Anopheles gambiae: From a complement-like protein to a complement-like pathway. Cell Host Microbe. 2008;3:364–374. doi: 10.1016/j.chom.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Levashina EA, et al. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 17.Fraiture M, et al. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Mitri C, et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009;5:e1000576. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Povelones M, et al. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habtewold T, et al. Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. PLoS Pathog. 2008;4:e1000070. doi: 10.1371/journal.ppat.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garver LS, et al. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 2009;5:e1000335. doi: 10.1371/journal.ppat.1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta L, et al. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meister S, et al. The Plasmodium parasite-a ‘new’ challenge for insect innate immunity. Int J Parasitol. 2004;34:1473–1482. doi: 10.1016/j.ijpara.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Garver LS, et al. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. 2013;9:e1003622. doi: 10.1371/journal.ppat.1003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt SM, et al. Use of a Drosophila model to identify genes regulating Plasmodium growth in the mosquito. Genetics. 2008;180:1671–1678. doi: 10.1534/genetics.108.089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyles DE. Studies on Plasmodium gallinaceum: IV. A comparison of the susceptibility of Aedes aegypti, Anopheles quadrimaculatus and Anopheles freeborni. Am J Epidemiol. 1952;56:71–77. [PubMed] [Google Scholar]
- 27.Haas VH, Akins H. Transmission of Plasmodium gallinaceum by Anopheles quadrimaculatus. J nat Malar Soc. 1947;6:144. [PubMed] [Google Scholar]
- 28.Hunninen AV. Comparative susceptibility of four anopheline mosquitoes to Plasmodium relictum. J Natl Malar Soc. 1951;10:216–223. [PubMed] [Google Scholar]
- 29.Collins FH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 30.Hume JCC, et al. Susceptibility of Anopheles stephensi to Plasmodium gallinaceum: A trait of the mosquito, the parasite, and the environment. PLoS ONE. 2011;6:e20156. doi: 10.1371/journal.pone.0020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunninen AV. Comparative development of Plasmodium relictum oocysts in Anopheles quadrimaculatus, A. albimanus, and Culex pipiens. J Parasitol. 1953;39:28–32. [PubMed] [Google Scholar]
- 32.Mok FF. Transmission of Plasmodium relictum Grassi & Feletti by Anopheles freeborni Aitken. Science. 1951;113:485–486. doi: 10.1126/science.113.2939.485. [DOI] [PubMed] [Google Scholar]
- 33.Gupta L, et al. Midgut epithelial responses of different mosquito-Plasmodium combinations: the actin cone zipper repair mechanism in Aedes aegypti. Proc Natl Acad Sci U S A. 2005;102:4010–4015. doi: 10.1073/pnas.0409642102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina-Cruz A, et al. Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes. Proc Natl Acad Sci USA. 2012;109:E1957–1962. doi: 10.1073/pnas.1121183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Lamballerie X, et al. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin E, et al. Differential responses of the mosquito Aedes albopictus from the Indian Ocean region to two Chikungunya isolates. BMC Ecol. 2010;10:8. doi: 10.1186/1472-6785-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsetsarkin KA, Weaver SC. Sequential adaptive mutations enhance efficient vector switching by Chikungunya virus and its epidemic emergence. PLoS Pathog. 2011;7:e1002412. doi: 10.1371/journal.ppat.1002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arias-Goeta C, et al. Dissemination and transmission of the E1-226V variant of Chikungunya virus in Aedes albopictus are controlled at the midgut barrier level. PLoS ONE. 2013;8:e57548. doi: 10.1371/journal.pone.0057548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno M, et al. Complete mtDNA genomes of Anopheles darlingi and an approach to anopheline divergence time. Malar J. 2010;9:127. doi: 10.1186/1475-2875-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joy DA, et al. Local adaptation and vector-mediated population structure in Plasmodium vivax malaria. Mol Biol Evol. 2008;25:1245–1252. doi: 10.1093/molbev/msn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez MH, et al. Different prevalences of Plasmodium vivax phenotypes VK210 and VK247 associated with the distribution of Anopheles albimanus and Anopheles pseudopunctipennis in Mexico. Am J Trop Med Hyg. 2000;62:122–127. doi: 10.4269/ajtmh.2000.62.122. [DOI] [PubMed] [Google Scholar]
- 42.Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. P Roy Soc Lon B Bio. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricklefs RE, et al. Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Syst Biol. 2004;53:111–119. doi: 10.1080/10635150490264987. [DOI] [PubMed] [Google Scholar]
- 44.Coene J. Malaria in urban and rural Kinshasa: the entomological input. Med Vet Entomol. 1993;7:127–137. doi: 10.1111/j.1365-2915.1993.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 45.Robert V, et al. Malaria transmission in urban sub-saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- 46.Klinkenberg E, et al. Impact of urban agriculture on malaria vectors in Accra, Ghana. Malar J. 2008;7:151. doi: 10.1186/1475-2875-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mourou JR, et al. Malaria transmission in Libreville: results of a one year survey. Malar J. 2012;11:40. doi: 10.1186/1475-2875-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subra R. Biology and control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Insect Sci Appl. 1981;1:319–338. [Google Scholar]
- 49.Trape JF, Zoulani A. Malaria and urbanization in central Africa: the example of Brazzaville. Part II: Results of entomological surveys and epidemiological analysis. Trans R Soc Trop Med Hyg. 1987;81:10–18. doi: 10.1016/0035-9203(87)90472-x. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly M, et al. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. doi: 10.1186/1475-2875-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White GB. WHO, editor. Geographical distribution of arthropod-borne diseases and their principal vectors. Geneva: 1989. Malaria; pp. 11–22. [Google Scholar]
- 52.Kiszewski A, et al. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70:486–498. [PubMed] [Google Scholar]
- 53.Russell PF, et al. Practical Malariology. W.B. Saunders Company; 1946. [Google Scholar]
- 54.Coatney GR, et al. The primate malarias. Government Printing Office; 1971. [Google Scholar]
- 55.Bray RS, Garnham PCC. Anopheles as vectors of animal malaria parasites. Bull World Health Organ. 1964;31:143–147. [PMC free article] [PubMed] [Google Scholar]
- 56.Warren M, Wharton RH. The vectors of simian malaria: Identity, biology, and geographical distribution. J Parasitol. 1963;49:892–904. [PubMed] [Google Scholar]
- 57.Cox FEG. Plasmodia of Rodents. In: Kreier JP, editor. Parasitic Protozoa. 5 Vol. 2. 1993. [Google Scholar]
- 58.Vargas L. Culicine and aedine mosquitoes and the malaria infections of lower animals. In: Boyd M, editor. Malariology. Saunders W.B; Philadelphia: 1949. pp. 526–538. [Google Scholar]
- 59.Diallo M, et al. Mosquito vectors of the 1998–1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med Vet Entomol. 2005;19:119–126. doi: 10.1111/j.0269-283X.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 60.Lutomiah JL, et al. Ability of selected kenyan mosquito (Diptera: Culicidae) species to transmit West Nile Virus under laboratory conditions. J Med Entomol. 2011;48:1197–1201. doi: 10.1603/me11062. [DOI] [PubMed] [Google Scholar]
- 61.Varma MGL, White GB. WHO, editor. Geographical distribution of arthropod-borne diseases and their principal vectors. Geneva: 1989. Mosquito Bourne Virus Diseases; pp. 35–54. [Google Scholar]
- 62.Ochieng C, et al. Mosquito-borne arbovirus surveillance at selected sites in diverse ecological zones of Kenya; 2007 – 2012. Virol J. 2013;10:140. doi: 10.1186/1743-422X-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brault AC, et al. Infection patterns of o’nyong nyong virus in the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol Biol. 2004;13:625–635. doi: 10.1111/j.0962-1075.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- 64.Monath TP, editor. The Arboviruses: ecology and epidemiology. CRC Press; 1988. [Google Scholar]
- 65.Vanlandingham DL, et al. Differential infectiveness of O’nyong-O’nyong and Chikungunya virus isolates in Anopheles gambiae and Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2005;72:616–621. [PubMed] [Google Scholar]
- 66.Yadav P, et al. Experimental transmisson of Chikungunya virus by Anopheles stephensi mosquitoes. Acta Virol. 2003:45–47. [PubMed] [Google Scholar]
- 67.White GB. WHO, editor. Geographical distribution of arthropod-borne diseases and their principal vectors. Geneva: 1989. Lymphatic filariasis; pp. 23–25. [Google Scholar]
- 68.Lefèvre T, et al. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 2013;9:e1003365. doi: 10.1371/journal.ppat.1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simon N, et al. Malaria parasites co-opt human factor H to prevent complement-mediated lysis in the mosquito midgut. Cell Host Microbe. 2013;13:29–41. doi: 10.1016/j.chom.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Cirimotich CM, et al. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34:387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langer RC, Vinetz JM. Plasmodium ookinete-secreted chitinase and parasite penetration of the mosquito peritrophic matrix. Trends Parasitol. 2001;17:269–272. doi: 10.1016/s1471-4922(01)01918-3. [DOI] [PubMed] [Google Scholar]
- 72.Molina-Cruz A, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. The Journal of biological chemistry. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira GdA, et al. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina-Cruz A, et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340:984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]