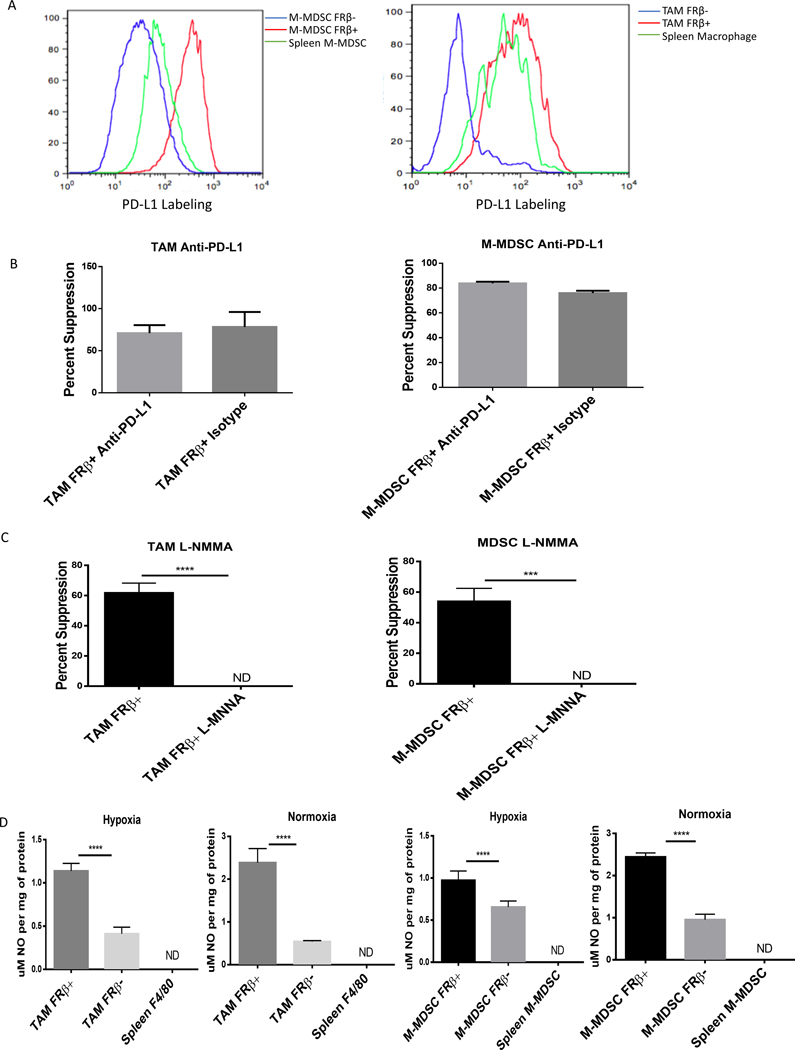
Figure 3: M-MDSCs and TAMs depend on nitric oxide production for immunosuppression.
(A) Expression of PD-L1 in tumor-derived FRβ+/− M-MDSCs and TAMs. IP RM-1 tumor exudates were stained with MDSC markers, anti-FRβ antibody, and an anti-PD-L1 antibody. FRβ+ M-MDSCs (red), FRβ− (blue) M-MDSCs, and spleen CD11b+ Ly-6C+ cells (green) were analyzed. (Data representative of 5 mice) (B) Impact of anti-PD-L1 blocking of PD-L1 on suppression of CD8 T cell proliferation mediated by tumor-derived MDSCs and TAMs (calculated as described in Materials and Methods). M-MDSCs and TAMs were isolated from IP RM-1 tumor bearing mice and divided into FRβ+/− subsets. Those cells were then tested in an 18 hr. suppression assay with antigen activated (SIINFEKL peptide 1 μg/mL) activated OT-1 T cells as described in Materials and Methods. Samples were treated with anti-PD-L1 antibody (10F.9G2) and suppression was compared to untreated control. (n=5 mice per group pooled with data representative of 2 experiments) (C) Impact of inhibiting nitric oxide production by tumor-derived MDSCs and TAMs on suppression. L-NMMA (50 μM), an inhibitor of nitric oxide production, was tested in the same assay as described in panel B. (n=5 mice per group pooled with data representative of 2 experiments) (D) Nitric oxide production in FRβ positive and negative M-MDSCs and TAMs. Tumor-derived and spleen M-MDSCs and TAMs were treated with IFNγ for 24 hrs. after isolation from IP RM-1 tumor bearing mice. Cells were lysed by freezing and lysates were tested with for nitric oxide production by Griess reagent. (n=5 mice per group pooled with data representative of 2 experiments)