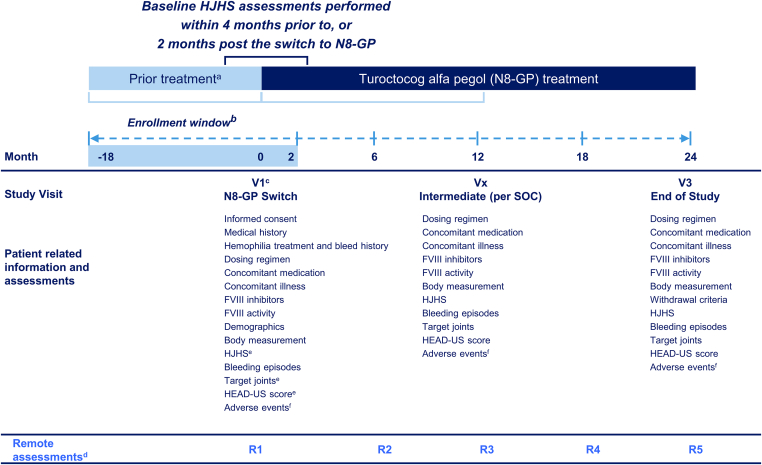
Figure.
pathfinderReal study design. aPrior treatment: any hemophilia treatment regime other than N8-GP received prior to study enrollment. bThe enrollment window: the date when the patient switched to N8-GP (within 18 months prior to enrollment) or planned to switch (within 2 months post enrollment) to prophylaxis with N8-GP from previous therapy. cVisit 1 may occur prior to enrollment due to criteria that allow patients who have switched to N8-GP in the 18 months prior to enrollment. dRemote PRO measurements: biannual remote direct data capture by participants is planned for the PRO assessments. eAssessments performed within 4 months prior to switch or 2 months after the switch to N8-GP can be used as baseline data. fSites should ask and report on adverse events at each contact with the patient during the course of the study. FVIII, factor VIII; HEAD-US, Hemophilia Early Arthropathy Detection with Ultrasound; HJHS, Hemophilia Joint Health Score; N8-GP, turoctocog alfa pegol; PRO, patient-reported outcome; Rx, remote assessment number; SOC, standard of care; Vx, visit number.