Abstract
Background:
Macrophages and microglia play critical roles after spinal cord injury (SCI), with the pro-healing, anti-inflammatory (M2) subtype being implicated in tissue repair. We hypothesize that promoting this phenotype within the post-injured cord microenvironment may provide beneficial effects for mitigating tissue damage. As a proof of concept, we propose the use of nanoparticles incorporating the carbohydrate antigen, galactose-α-1,3-galactose (α-gal epitope) as an immunomodulator to transition human microglia (HMC3) cells toward a pro-healing state.
Methods:
Quiescent HMC3 cells were acutely exposed to α-gal nanoparticles in the presence of human serum and subsequently characterized for changes in cell shape, expression of anti or pro-inflammatory markers, and secretion of phenotype-specific cytokines.
Results:
HMC3 cells treated with serum activated α-gal nanoparticles exhibited rapid enlargement and shape change in addition to expressing CD68. Moreover, these activated cells showed increased expression of anti-inflammatory markers like Arginase-1 and CD206 without increasing production of pro-inflammatory cytokines TNF-α or IL-6.
Conclusion:
This study is the first to show that resting human microglia exposed to a complex of α-gal nanoparticles and anti-Gal (from human serum) can be activated and polarized toward a putative M2 state. The data suggests that α-gal nanoparticles may have therapeutic relevance to the CNS microenvironment, in both recruiting and polarizing macrophages/microglia at the application site. The immunomodulatory activity of these α-gal nanoparticles post-SCI is further described in the companion work (Part II).
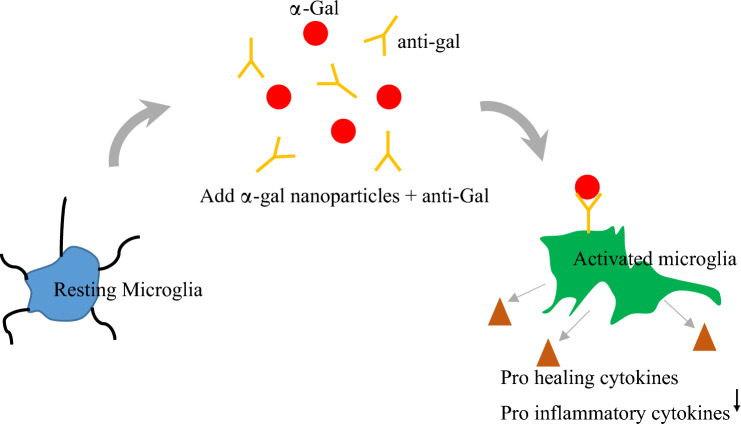
Graphical abstract
Resting microglia subjected to α-gal nanoparticle treatment in the presence of anti-Gal (found in serum) become activated and exhibit pro-healing phenotypic markers (Arginase-1, CD206) and secrete VEGF. Expression of pro-inflammatory markers (IL-6, TNF-α) was concomitantly reduced.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-023-00613-1.
Keywords: Microglia, α-gal, Anti-inflammatory, Immunomodulation, Cytokines, Spinal cord injury
Introduction
Following traumatic spinal cord injury (SCI), macrophage populations migrate to the injury milieu via the hemorrhagic lesion. These macrophages perform many functions, such as phagocytosis of cellular debris, antigen presentation, homeostatic maintenance, and secretion of multiple pro and anti-inflammatory cytokines [1, 2]. Blood-borne macrophages are further supported by microglia, which are resident macrophages within the CNS. Microglia function as sentinels to detect foreign agents or injury byproducts and become quickly activated after SCI [3]. Like blood-derived macrophages, microglia are commonly classified by their functional pro-inflammatory (M1) or anti-inflammatory (M2) states [4]. Microglia that are CD11b+/CD45low and IL-12high, IL-10low, CD14high/CD16− are characteristic of the pro-inflammatory M1 phenotype. They are activated by iNOS, IFN‐γ, TNF‐α, LPS, and IL-12 [5–7]. On the other hand, the anti-inflammatory M2 state is identified as IL-10high, IL-12low, CD14low/CD16+ and is activated by TGF-β, IL‐18, IL‐10, and IL-4 [6, 7]. Interestingly, both macrophages and microglia arise from yolk sac myeloid progenitor cells [8, 9]. The shared genesis and phenotypic similarity between these two lineages suggest they may also respond comparably to stimuli, such as exogenous agents that initiate Fcγ-receptor (FcγR) binding. We evaluated this hypothesis by stimulating human microglia with immune complexes formed between the natural anti-Gal antibody and nanoparticles composed of α-gal epitopes (in the form of α-gal decorated nanoparticles).
The α-gal epitope is a carbohydrate antigen with the structure Galα1-3Galβ1-4GlcNAc-R that is synthesized in non-primates and New-World monkeys by the glycosylation enzyme α1,3galactosyltransferase [10]. Humans, apes, and Old-World monkeys lack this enzyme and do not synthesize α-gal epitopes, but produce a natural antibody called anti-Gal, which binds the α-gal epitope [11, 12]. Anti-Gal is abundantly produced in humans as a natural antibody, constituting ~ 1% of circulating antibodies [13] in response to carbohydrate antigens found in gastrointestinal bacteria [14]. In vivo, the strong interaction between anti-Gal and exogenous α-gal epitopes causes a two-fold immunologic response. First, this antigen–antibody interaction activates the complement system and releases macrophage chemo-attractants complement cleavage peptides (such as C5a and C3a) [15–17]. Secondly, the anti-Gal/α-gal immune complex activates macrophages by binding the Fc “tail” of the immunocomplex antibody to the Fcγ receptor on the macrophages. The rapidity of α-gal binding and the subsequent immunologic cascade have been well documented in xenotransplants (i.e., transplantation of porcine tissues and organs into monkeys or humans). In such instances, the binding of anti-Gal to α-gal epitopes on endothelial cells of the porcine blood vessels initiates the complement cascade that destroys these cells, resulting in rapid (hyperacute) rejection of the xenograft [18–21].
In contrast to the exposure of α-gal epitopes to anti-Gal in the circulation, the physiologic response to the localized α-gal application is markedly different. In both pig and transgenic mouse wound healing models, α-gal epitopes conjugated to phospholipid nanoparticles (α-gal nanoparticles) shortened healing times by 40–50% without fibrotic scarring [16, 22]. Application of α-gal nanoparticles to wounds induces macrophage chemotaxis through C5a and C3a complement cleavage peptides. Further, the Fc/FcγR interaction stimulated the macrophages to produce factors such as VEGF, PDGF, and CSF1, which promote tissue repair [10, 16, 17]. Likewise, intramyocardial injection of α-gal nanoparticles into anti-Gal producing mice with myocardial infarction resulted in a ten-fold reduction in scar size and restoration of contractile function [15, 23]. While the phenotype of these beneficial macrophages was not elucidated, the results suggest pro-healing macrophages may have been recruited to the injection sites.
In the present work, our goal was to explore the microglial response to α-gal nanoparticles and to determine whether microglia can be polarized into a pro-healing phenotype similar to blood borne macrophages. We exposed HMC3 microglia to α-gal nanoparticles in the presence of anti-Gal (i.e., from human serum), and a combination of flow cytometry, cell morphology, and ELISA was used to subsequently characterize microglia phenotype. Since microglia are resident immune cells of the CNS, the experimental results have implications for SCI.
Materials and methods
Materials
HMC3 (Human Microglia, ATTC-CRL3304) cells were purchased from ATCC. Tissue culture materials such as DMEM, fetal bovine serum (FBS), and trypsin were purchased from Sigma-Aldrich. 4% paraformaldehyde was purchased from Thermo Fisher. DAPI was purchased from Biotium, and Dylight 554 phalloidin was purchased from LifeTechnologies. Antibodies such as CD206-PE and Arginase-1-PE were purchased from BioLegend and BS-lectin-FITC from Vector Labs. Promega NO detection kit was purchased for the nitric oxide (NO) assay. ELISA kits for IL-6 and TNF-α were purchased from Biolegend or Abcam for VEGF.
Characterization of nanoparticles
The α-gal nanoparticles were formed as previously described using glycolipids and phospholipids extracted from rabbit red blood cell (RBC) membranes [16, 22]. These nanoparticles were studied for their size, polydispersion index, and zeta potential. The α-gal nanoparticles at a concentration of 0.5 µg/mL were mixed with 1 mM KCl buffer (pH 7.4), and the resultant sample was analyzed using Zetasizer nano-ZS90 at 633 nm. For size and poly dispersion index (PDI) measurements, 0.5 µg/mL α-gal nanoparticles were mixed with 1X PBS and analyzed at 633 nm using a Zetasizer nano-ZS90. All experiments were performed in triplicate and the average recorded.
Transmission electron microscopy (TEM)
Glow discharge was performed using 400-mesh formvar copper grids. 2 µl of α-gal nanoparticles was pipetted onto the coated grid held in self-locking tweezers. The sample was allowed to dry at RT for 1 min. After evaporation, any excess liquid was wicked using the filter paper edge. Additionally, 2 µl of uranyl acetate or phosphotungstic acid was added as negative staining onto the grid. Excess uranyl acetate or phosphotungstic acid was wicked again with a filter paper edge, and the sample was transferred into the TEM. The nanoparticles were imaged on an FEI T12 TEM (Hillsboro, OR, USA) using an 80 kV accelerating voltage.
Cell culture and maintenance
HMC3 cells were cultured using a T75 cm2 flask in DMEM medium with 1 g/L D-glucose and 4 mM L-glutamine and supplemented with 10% FBS at 37 °C in a humidified atmosphere of 5% CO2 and 95% O2, with subculturing at 80%-90% confluence. All cell culture experiments were used between passages P3 to P6.
Preparation of α-gal nanoparticles/anti-Gal immune complex
One µl of 100 mg/mL of α-gal nanoparticles was initially mixed with 20 µl of human serum for 1 h sonication. Anti-Gal antibodies within the human serum immediately bind to the α-gal epitopes, creating an anti-Gal/α-gal nanoparticle complex. The samples were then centrifuged, and the resultant pellets were then resuspended with 1X PBS to obtain a final concentration of 10 mg/mL of nanoparticles. The final mixture was sonicated for about 30 s before being added to the cells.
Treatment of HMC3 cells with α-gal nanoparticles
HMC3 cells were seeded at a density of 50,000 cells per well in a 24-well plate and cultured up to 70% confluence. Cells were subdivided into four exposure conditions for 3 h: (1) HMC3 cells treated with the α-gal nanoparticles + human serum (2) HMC3 cells exposed to just α-gal nanoparticles with no serum, or (3) serum only, and (4) media control. After 3 h, the treated cells were washed with 1X PBS, fixed with 1% paraformaldehyde, and further analyzed.
Fluorescence microscopy
HMC3 cells were treated with either media alone, α-gal nanoparticles, or an α-gal/anti-Gal complex. The resultant cells were washed with 1X PBS two times and then incubated with 15 µl of Bandeiraea (Griffonia) simplicifolia IB4 (BS-lectin binds to α-gal nanoparticles) for 30 min at 37 °C, which were followed by nuclei staining with DAPI (1 µM) for 1 h. Additionally, for imaging of F-actin, the treated cells were fixed with 4% paraformaldehyde, and Dylight 554 phalloidin and DAPI (1 µM) were added for 30 min at room temperature. Images were then captured by an Olympus IX8 microscope and fluorescence intensities of 100 cells from different fields were calculated using the method described above. Results were presented as mean ± SE from three independent experiments (n = 3).
Morphological quantitation of HMC3 cells
Changes to microglia morphology were analyzed from photomicrographs of stained cells. Shape parameters such as total area, elongation, and circularity were tabulated using NIS Elements software (Version 5.02.00). Cell area was measured as cell size, and it is expressed as the number of pixels. Circularity was measured using the formula ((4πArea/(Perimeter)2) and a complete circle is represented as one. Cell elongation is measured as the maximum Feret to minimum Feret ratio. A single nuclei cell was used for analysis.
Flow cytometry
Flow cytometry was performed as previously described [24]. Briefly, the α-gal nanoparticle- treated HMC3 cells were suspended in a flow buffer (1X PBS plus 1% FBS) and incubated with indicated fluorochrome-labeled antibodies at 4 °C for 30 min and washed according to the manufacturer’s instructions. The antibodies were CD206, Arginase-1-PE, and BS-lectin-FITC. The samples were run on a BD FACS Calibur (Becton Dickinson, Franklin Lakes, NJ, USA) with excitation at 488 nm and detection at 575/30 nm for the PE channel, excitation at 494 nm, and detection at 520 nm for the FITC channel. The data recorded were analyzed using Kaluza 1.2 software (Beckman Coulter). Live-positive BS lectin was used as a gating strategy for quantifying the cells that express anti-inflammatory markers.
Nitric oxide (NO) measurement
The expression of NO was evaluated using the Griess assay. In brief, ~ 10,000 cells were seeded per well in a 96-well plate. The wells were treated with or without α-gal nanoparticles and incubated with anti-Gal. The supernatant was then collected at 12 h, 24 h, and 48 h. The concentration of NO was assessed using a manufacturer’s protocol from a commercially available Promega NO detection kit.
ELISA
The secretion of various cytokines like TNF-α, IL-6, and VEGF was evaluated by ELISA. In brief, 10,000 cells are seeded per well in a 96-well plate and treated with or without serum-incubated α-gal nanoparticles for 24 h and 48 h. Additionally, cells were stimulated with IFN-γ (10 ng/mL) for the above-mentioned time points. The supernatant was collected at respective time points and 25 µl of the sample aliquots were used for evaluating the expression of cytokines such as IL-6 and TNF-α or VEGF. The concentration of cytokines was then determined.
Statistics
All results are representative of at least three independent experiments. Data were analyzed by one-way ANOVA and post hoc tests, using Graph Pad 9.0 (Graph Pad Software, CA, USA). p-values < 0.05 were considered to be statistically significant. For non-parametric data, Kruskal–Wallis analysis (one-way ANOVA) was performed using Graph Pad 9.0.
Results
Characteristics of α-gal nanoparticles
Native α-gal nanoparticles were found to be quite varied in terms of size, with a polydispersity index (PDI) of 0.988. This was validated with TEM images (Fig. 1A). The mean particle size was found to be 317.8 ± 24.8 nm (Fig. 1B). Additionally, the nanoparticles were negatively charged, with a zeta potential of −27.5 ± 10.8 mV (Fig. 1B). The negative zeta potential is likely from the negatively charged sialic acid present on rabbit RBC membranes.
Fig. 1.
Characteristics of typical α-gal nanoparticles. A TEM image depicting native α-gal nanoparticles. Scale: 100nm B Table of α-gal nanoparticle physical properties
Binding of α-gal/anti-Gal nanoparticles to HMC3 cells
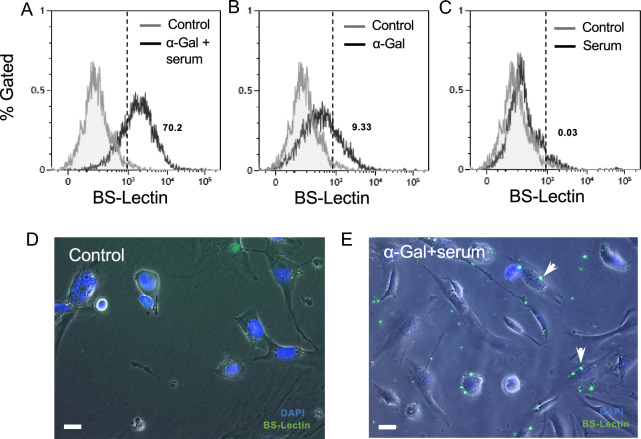
To quantify and visualize the binding of the anti-Gal/α-gal nanoparticle complex to HMC3 cells (human cells lacking α-gal epitopes), we used a fluorescent FITC conjugated BS-lectin marker to label the nanoparticles. BS-Lectin is a glycoprotein that binds to α-gal epitopes. Tested culture conditions were: (1) HMC3 cells incubated with human serum only, (2) HMC3 incubated with nanoparticles only, and (3) HMC3 with nanoparticles and human serum added. An increase in fluorescence intensity was observed in HMC3 cells exposed to the immune complex (α-gal nanoparticles and serum) (70.2% gated cells) (Fig. 2A) compared to control HMC3 cells via flow cytometry. Similarly, fluorescent images showed higher fluorescence intensity and localized staining of the nanoparticles on the cell surface vs control (Fig. 2D and E). Furthermore, when cells were incubated with either nanoparticles or serum only (Fig. 2B and C), the median fluorescence intensity (MFI) was similar to the media control. These findings suggest that the increased fluorescence signal was not due to the presence of α-gal nanoparticles alone but rather to the bound nanoparticle complex. These results collectively suggest that the α-gal nanoparticles only bind to HMC3 cells when the nanoparticles are coated with anti-Gal (i.e., “activated”) found in human serum.
Fig. 2.
Binding of α-gal nanoparticles to HMC3 cells under different treatment conditions. A FITC conjugated BS-Lectin was used to fluorescently label α-gal nanoparticles. Representative flow cytometry spectrograms of HMC3 cells incubated with α-gal nanoparticles and human serum (immune complex) or B cells incubated with α-gal nanoparticles only or C cells incubated with human serum only. Also shown are respective control groups (HMC3 in media only). Fluorescence microscopy at 10X was used for visual confirmation of nanoparticle binding D. Control and E immune complex. White arrows denote nanoparticle binding of BS-lectin. Scale: 100µm. The dotted vertical line represents the median fluorescence intensity (MFI) shift for BS-lectin
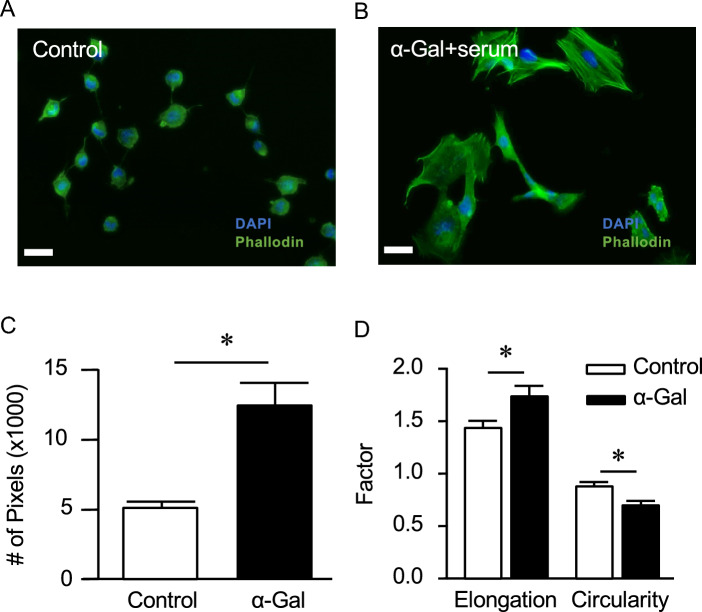
Morphological change to HMC3
When HMC3 cells were exposed to the α-gal/anti-Gal nanoparticle complex, the cells assumed a very different morphology (Fig. 3B) vs the resting (control) state (Fig. 3A), as visualized by phase contrast and fluorescence microscopy (F-actin staining). The control cells possessed a small soma with branches and were more circular (Fig. 3C). In contrast, the nanoparticle-treated cells were over three times larger and much more elongated (p < 0.05, Fig. 3C, D). Likewise, the circularity index of cells decreased with α-gal nanoparticle treatment (p < 0.05, Fig. 3D). F-actin staining was also more prominent in the treated cells, corresponding to the increase in cell size.
Fig. 3.
Immunofluorescent images of HMC3 cells in the A untreated state or B exposure to α-gal nanoparticle complex. A rapid change in cell morphology was observed after α-gal complex treatment. Morphological measurements depict a significant increase in the cell area C, elongation and a decrease in circularity D. *p < 0.05 vs. control. Scale: 100µm
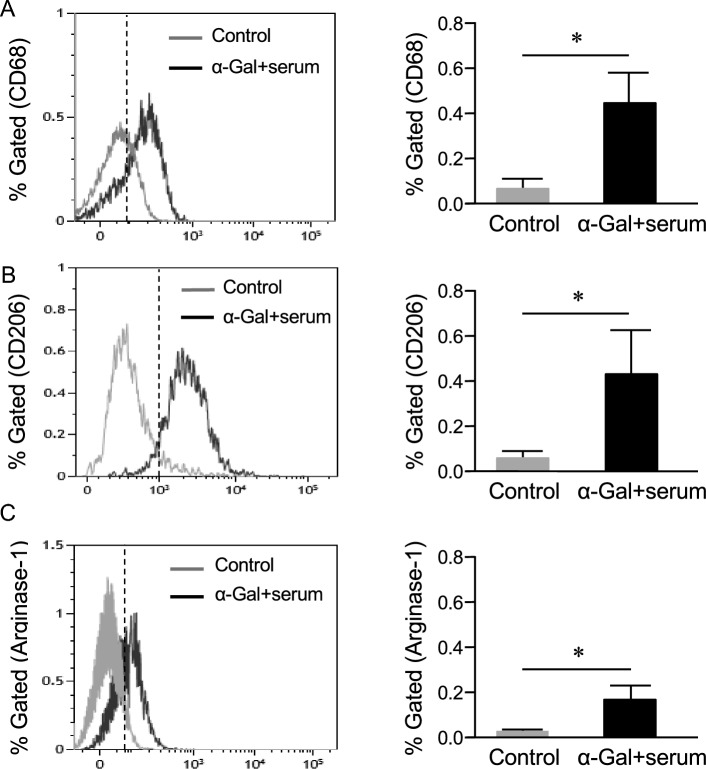
Expression of CD68 and anti-inflammatory markers by α-gal nanoparticles in HMC3 cells
CD68, a pan-phagocytic marker that is expressed by activated microglia [25], was used to screen cells via flow cytometry. As received, there was no CD68 expression in control HMC3s, suggesting a resting state. However, when cells were incubated with α-gal nanoparticles and serum, a shift towards a statistically significantly higher CD68 expression was observed (Fig. 4A , p < 0.05). Additionally, Arginase-1 and CD206 (anti-inflammatory markers) were used to assess the HMC3 state. Results show that anti-Gal/α-gal nanoparticle immune complexes induced the expression of Arginase-1 compared to control cells (Fig. 4C , p < 0.05). Similarly, CD206 expression was higher in cells incubated with bound anti-Gal/α-gal nanoparticle immune complexes compared to control cells (Fig. 4B , p < 0.05). Note that the expression of CD68, CD206, and Arginase-1 did not change in cells incubated with serum or α-gal nanoparticles alone (Fig. S1). Since there were no such changes, subsequent experiments only used the anti-Gal/α-gal nanoparticle immune complexes as the treatment.
Fig. 4.
Flow cytometry characterization of cellular markers expressed by HMC3 cells. HMC3 cells were treated with anti-Gal/α-gal nanoparticles immune complex (3 h) and compared with untreated controls for A CD68 B CD206 or C Arginase-1 expression. Data showed a significant shift in the median fluorescence intensity (MFI) of these surface markers. The markers are expressed as live singlets using BS-lectin positive cells. Corresponding plots quantifying percent gated cells are shown (mean ± SE, *p < 0.05 vs. control, n = 3 runs). The dotted vertical line represents the median fluorescence intensity (MFI) shift for markers
Expression of cytokine markers by activated HMC3 cells
The pro-inflammatory cytokines IL-6 and TNF-α, as well as the angiogenic factor vascular endothelial growth factor (VEGF), were quantified using ELISA. We found that control cells produced 295 ± 12 pg/mL IL-6 after 24 h and upon stimulation with IFN-γ, the IL-6 expression increased to 346 ± 27 pg/mL. However, when HMC3 cells were incubated with the anti-Gal/α-gal nanoparticle immune complexes, the IL-6 expression decreased significantly to 225 ± 9 pg/mL vs cells incubated with IFN-γ (p < 0.01, Fig. 5B). Similarly, after 48 h post-treatment with the immune complexes, the IL-6 expression decreased significantly compared to IFN-γ incubated cells (p < 0.01, Fig. 5B). Contrastingly, the pro-inflammatory marker TNF-α increased slightly with IFN-γ treatment at 24 h (8 ± 2.4 pg/mL) but was reduced to 6 ± 1.1 pg/mL with the immune complexes (p < 0.05, Fig. 5A). Likewise, after 48 h IFN-γ treatment, TNF-α did not increase compared to control cells and there was no decrease in expression when cells were incubated with the immune complexes (Fig. 5A). Correspondingly, the anti-inflammatory vascular endothelial growth factor (VEGF) expression did not change for cells incubated with the immune complex (5.6 ± 0.25 pg/mL) vs control (5.3 ± 0.36 pg/mL) or cells incubated with IFN-γ (5.3 ± 0.38 pg/mL) after 24 h (Fig. 5C). After 48 h, we found a slight increase in VEGF expression (6.5 ± 0.41 pg/mL) compared to control cells or cells with IFN-γ treatment, but was not statistically significant (Fig. 5C). Similarly, iNOS (a pro-inflammatory marker and a precursor to NO) was used to assess the HMC3 state, and no change in iNOS was found in any of the treatment regimens (Fig. 5D).
Fig. 5.
Expression of various cytokines in HMC3 cells. The cells were treated with IFN-γ (10 ng/mL) or with anti-Gal/α-gal nanoparticles immune complexes (10 mg/mL) for up to 48 h. The supernatants were collected at respective time points and the levels of A TNF-α B IL-6 or C VEGF were measured using ELISA. D NO generation by HMC3 cells at different times post-treatment. *p < 0.1, **p < 0.05, ***p < 0.01 n = 3 runs
Discussion
After SCI, resident CNS microglia play a major role in pathogen and damage-associated phagocytosis, removal of devitalized tissue, and secretion of cytokines necessary for repair and remodeling [26]. Like their blood-derived counterparts, such disparate functions of microglia are related to the phenotype, traditionally categorized as either pro-inflammatory or anti-inflammatory (pro-healing). M1 subtype macrophages are present during the early phase of SCI repair [26, 27]. M1 macrophages release pro-inflammatory cytokines that attract neutrophils to clear damaged tissue [28–31]. Other M1 products include reactive oxygen species (ROS), IL-8, and TNF-α, which activate neutrophils and recruit additional macrophages for debris removal [32]. In contrast, M2 anti-inflammatory macrophages reduce ROS production and pro-inflammatory cytokines like TNF-α and IL-6 [33, 34]. These macrophages also facilitate debris scavenging and upregulate pro-healing chemokine and cytokine production [26, 35]. The deficient injury resolution post-SCI may be associated with the dysfunctional transition from the M1 to M2 phase in CNS macrophages/microglia [6, 26, 31]. Some studies have shown that modulating the phenotype by directly exposing microglia to anti-inflammatory cytokines such as IL-10, IL-4, and IL-13 can provide a therapeutic benefit [36]. For instance, Lee et al. showed that the addition of IL-4 promoted axonal growth, while depletion of IL-4 in mice resulted in increased damage after SCI [37]. Similarly, the addition of IL-10 can enhance the anti-inflammatory response [6, 38], while its reduction results in overexpression of the pro-inflammatory microglia phenotype [39, 40]. However, inherent problems of cytokine therapy are cross-talk between different cell signaling pathways, potential systemic effects, and diffusion away from the treated site, which causes a decrease in the effective concentration of the cytokine within short periods post-treatment.
Here, we propose a simple approach to the spatiotemporal manipulation of macrophage phenotype by exploiting innate immunity and the natural response to the α-gal epitope. In previous studies, α-gal nanoparticles were used to locally recruit pro-healing macrophages to the injury site [16, 22]. These α-gal nanoparticles bind to anti-Gal antibodies found in serum that has leaked from ruptured capillaries. The formed anti-Gal/α-gal nanoparticle immune complexes mediate the release of complement cleavage chemotactic peptides through the initiation of the complement cascade. The chemotactic cues recruit additional macrophages, and the anti-Gal/α-gal complex further binds to the recruited macrophages via Fc/FcγR interactions to activate these macrophages [17, 41]. In mouse models of dermal and myocardial injury, these activated macrophages purportedly secrete key cytokines and growth factors necessary for tissue healing and remodeling [15, 22, 42].
Our results with HMC3 cells suggest that the α-gal/anti-Gal immunocomplex may induce a similar activation in human microglia. The α-gal/anti-Gal complex readily attach to HMC3 cells, and this binding was accompanied by a rapid shape change from a branched, rounded form to an ameboid shape (reduced circularity) with pronounced F-actin staining (Fig. 3B). Others have shown that HMC3 also changes from a resting ramified morphology to an amoeboid morphology when stimulated with LPS [43, 44]. This shape change appears to be a precursor to cell polarization or differentiation [45]. Further flow cytometry screening identified the immunocomplex treated cells as CD68-positive. In the resting state, HMC3s are typically negative for CD68, MHCII, CD11b, and GFAP but positive for IBA-1 and CD14 [25, 46]. The expression of CD68 supports an activated microglia state [25, 46]. In contrast, bare α-gal nanoparticles did not produce any appreciable increase in cell surface fluorescence, shape or size change, or CD68 expression. This finding was also consistent with HMC3 cells exposed to serium only, demonstrating that the Fc “tail” on the α-gal/anti-Gal immunocomplex is responsible for both cell binding and subsequent microglia activation.
Several biomarkers were used to assess the activated HMC3 phenotype and function. Both Arginase-1 and CD206 increased upon α-gal/anti-Gal immunocomplex treatment (Fig. 4). These markers have been shown to exist in pro-healing macrophage populations [47]. The findings in microglia are also similar to prior studies whereby subcutaneous delivery of α-gal nanoparticles from sponge discs attracted macrophages expressing high levels of IL-10 and Arginase-1 and low levels of IL-12 [48]. These recruited macrophages were implicated in faster wound closure and enhanced wound healing in a diabetic mouse model. We note that HMC3 cells exposed to serum or α-gal nanoparticles alone did not significantly increase CD206, Arginase-1, or CD68 expression (Fig. S1). This again suggests that for the nanoparticles to become bioactive, they must first bind to anti-Gal found in serum.
Since the α-gal immunocomplex may potentially act as a pan-activating agent and drive HMC3 cells towards the M1 path, we additionally quantified the pro-inflammatory products NO, TNF-α, and IL-6. IFN-γ was used as a positive control since it is a common pro-inflammatory induction agent in macrophages [49]. Unstimulated HMC3 cells produced IL-6 at a high basal level (~ 280–300 pg/mL) which is consistent with other studies [25, 50]. However, IL-6 production at 24 h and 48 h in the immune complex groups was similar to controls and much lower than IFN-γ. TNF-α was also lower at 24 h with anti-Gal/α-gal nanoparticle complexes vs the IFN-γ cohort. Interestingly, NO values were about the same regardless of the stimulation regime (Fig. 5D). It has been reported that exposure to anti-Gal coated α-gal nanoparticles increased the macrophage production of certain cytokines (such as PDGF, CSF1, or VEGF) that may have enhanced wound healing [16]. We investigated VEGF secretion with ELISA and noticed the values trended higher for the immune complex treatment at longer exposure times (48 h) vs control and IFN-γ. It is unclear if a longer sampling period would have resulted in higher VEGF values.
As a whole, HMC3 cells expressed several M2 surface markers, although some expected functions of M2 microglia were not observed. This may be a limitation of the HMC3 line. HMC3 cells uniquely express the NOX4 gene constitutively and produce iNOS and IL-6 even in the resting state [25]. This may explain the invariant NO values under the different test conditions. Likewise, cytokines such as VEGF may be expressed predominately at the mRNA level instead of at the protein level [46]. Future studies will need to be conducted to confirm these traits via gene expression microarrays. Nonetheless, the results show that anti-Gal/α-gal nanoparticle immune complexes did not promote polarization towards the M1 state, especially when compared to IFN-γ, a pro-inflammatory agent.
In conclusion, this is the first study to show that anti-Gal/α-gal nanoparticle immune complexes activate human microglia cells and drive them toward a putative M2 state. Activated CD68 positive HMC3 cells showed increased F-actin synthesis and cell size, CD206 and Arginase-1 expression, and decreased IL-6 expression. A slight trend towards increased VEGF secretion was also found. The observed immune-modulatory results with activated α-gal nanoparticles are comparable to what has been reported with blood-derived macrophages. This directed microglia polarization towards the pro-healing phenotype may be beneficial after SCI. In such instances, the application of α-gal nanoparticles may recruit and polarize both infiltrated macrophages and resident microglia. We subsequently elucidate the in vivo activity of α-gal nanoparticles in modulating the reparative response post-SCI in the companion work.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was partly funded by the State of Indiana and Clinical and Translational Sciences Institute (CTSI, Indiana State Department of Health (Grant # 204200 to JL) and National Institute of Neurological Disorders and Stroke R21 (No. 1R21NS115094-01).
Funding
Indiana Clinical and Translational Sciences Institute (204200), National Institute of Neurological Disorders and Stroke (1R21NS115094-01).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have not disclosed any conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. doi: 10.3389/fncel.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman D. Regulation of macrophage phagocytosis. Eur J Clin Microbiol. 1986;5:1–5. doi: 10.1007/BF02013451. [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 4.Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 5.Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci. 2014;8:461. doi: 10.3389/fncel.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflamm. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurga AM, Paleczna M, Kuter KZ. Overview of general and discriminating markers of differential microglia phenotypes. Front Cell Neurosci. 2020;14:198. doi: 10.3389/fncel.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samokhvalov IM, Samokhvalova NI, Nishikawa S-I. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 9.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–80. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 10.Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140:1–11. doi: 10.1111/imm.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1–3Gal epitope in primates. Proc Natl Acad Sci USA. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and old world monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. doi: 10.1016/S0021-9258(19)77900-9. [DOI] [PubMed] [Google Scholar]
- 13.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56:1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galili U, Zhu Z, Chen J, Goldufsky JW, Schaer GL. Near complete repair after myocardial infarction in adult mice by altering the inflammatory response with intramyocardial injection of alpha-Gal nanoparticles. Front Cardiovasc Med. 2021;8:719160. doi: 10.3389/fcvm.2021.719160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigglesworth KM, Racki WJ, Mishra R, Szomolanyi-Tsuda E, Greiner DL, Galili U. Rapid recruitment and activation of macrophages by anti-Gal/alpha-Gal liposome interaction accelerates wound healing. J Immunol. 2011;186:4422–4432. doi: 10.4049/jimmunol.1002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galili U. Antibody production and tolerance to the alpha-gal epitope as models for understanding and preventing the immune response to incompatible ABO carbohydrate antigens and for alpha-gal therapies. Front Mol Biosci. 2023;10:1209974. doi: 10.3389/fmolb.2023.1209974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–482. doi: 10.1016/0167-5699(93)90261-I. [DOI] [PubMed] [Google Scholar]
- 19.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transpl Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 20.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1–3)Gal epitopes. Proc Natl Acad Sci USA. 1993;90:11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins BH, Cotterell AH, McCurry KR, Alvarado CG, Magee JC, Parker W, et al. Cardiac xenografts between primate species provide evidence for the importance of the alpha-galactosyl determinant in hyperacute rejection. J Immunol. 1995;154:5500–5510. doi: 10.4049/jimmunol.154.10.5500. [DOI] [PubMed] [Google Scholar]
- 22.Galili U, Wigglesworth K, Abdel-Motal UM. Accelerated healing of skin burns by anti-Gal/α-gal liposomes interaction. Burns. 2010;36:239–251. doi: 10.1016/j.burns.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Galili U. Biosynthesis of alpha-Gal epitopes (Galalpha1-3Galbeta1-4GlcNAc-R) and their unique potential in future alpha-Gal therapies. Front Mol Biosci. 2021;8:746883. doi: 10.3389/fmolb.2021.746883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vasu S, He S, Cheney C, Gopalakrishnan B, Mani R, Lozanski G, et al. Decitabine enhances anti-CD33 monoclonal antibody BI 836858-mediated natural killer ADCC against AML blasts. Blood. 2016;127:2879–2889. doi: 10.1182/blood-2015-11-680546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Bedard K, Sorce S, Hinz B, Dubois-Dauphin M, Krause KH. NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL-6 expression. J Innate Immun. 2009;1:570–581. doi: 10.1159/000235563. [DOI] [PubMed] [Google Scholar]
- 26.Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W, Li JM. Targeted siRNA delivery reduces nitric oxide mediated cell death after spinal cord injury. J Nanobiotechnol. 2017;15:1–11. doi: 10.1186/s12951-017-0272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801. doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, et al. M1/M2 macrophages and their overlaps–myth or reality? Clin Sci. 2023;137:1067–1093. doi: 10.1042/CS20220531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zivkovic S, Ayazi M, Hammel G, Ren Y. For better or for worse: a look into neutrophils in traumatic spinal cord injury. Front Cell Neurosci. 2021;15:648076. doi: 10.3389/fncel.2021.648076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep. 2017;7:40144. doi: 10.1038/srep40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong XY, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–954. doi: 10.1111/jcmm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Broeckhoven J, Erens C, Sommer D, Scheijen E, Sanchez S, Vidal PM, et al. Macrophage-based delivery of interleukin-13 improves functional and histopathological outcomes following spinal cord injury. J Neuroinflamm. 2022;19:1–19. doi: 10.1186/s12974-022-02458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SI, Jeong SR, Kang YM, Han DH, Jin BK, Namgung U, et al. Endogenous expression of interleukin-4 regulates macrophage activation and confines cavity formation after traumatic spinal cord injury. J Neurosci Res. 2010;88:2409–2419. doi: 10.1002/jnr.22411. [DOI] [PubMed] [Google Scholar]
- 38.Zhou ZG, Peng XM, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol. 2009;220:183–190. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laffer B, Bauer D, Wasmuth S, Busch M, Jalilvand TV, Thanos S, et al. Loss of IL-10 promotes differentiation of microglia to a M1 phenotype. Front Cell Neurosci. 2019;13:430. doi: 10.3389/fncel.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren Y, Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013;2013:945034. doi: 10.1155/2013/945034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galili U. alpha-Gal nanoparticles in wound and burn healing acceleration. Adv Wound Care. 2017;6:81–92. doi: 10.1089/wound.2016.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurwitz ZM, Ignotz R, Lalikos JF, Galili U. Accelerated porcine wound healing after treatment with α-gal nanoparticles. Plast Reconstr Surg. 2012;129:242e–e251. doi: 10.1097/PRS.0b013e31823aebb1. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Contreras M, Thakor AS. Human adipose tissue-derived mesenchymal stem cells and their extracellular vesicles modulate lipopolysaccharide activated human microglia. Cell Death Discov. 2021;7:98. doi: 10.1038/s41420-021-00471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munoz Herrera OM, Hong BV, Ruiz Mendiola U, Maezawa I, Jin LW, Lebrilla CB, et al. Cholesterol, amyloid beta, fructose, and LPS influence ROS and ATP concentrations and the phagocytic capacity of HMC3 human microglia cell line. Int J Mol Sci. 2023;24:10396. doi: 10.3390/ijms241210396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dello Russo C, Cappoli N, Coletta I, Mezzogori D, Paciello F, Pozzoli G, et al. The human microglial HMC3 cell line: where do we stand? A systematic literature review. J Neuroinflamm. 2018;15:1–24. doi: 10.1186/s12974-018-1288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng YJ, Chen YS, Lin CW, Shen TL, Mersmann HJ, Ding ST. Docosahexaenoic acid suppresses pro-inflammatory macrophages and promotes anti-inflammatory/regulatory macrophage polarization through regulation of cytokines. Int J Clin Exp Med. 2018;11:10788–94.
- 48.Kaymakcalan OE, Abadeer A, Goldufsky JW, Galili U, Karinja SJ, Dong X, et al. Topical alpha-gal nanoparticles accelerate diabetic wound healing. Exp Dermatol. 2020;29:404–413. doi: 10.1111/exd.14084. [DOI] [PubMed] [Google Scholar]
- 49.Lu S, Li D, Xi L, Calderone R. Interplay of interferon-gamma and macrophage polarization during Talaromyces marneffei infection. Microb Pathog. 2019;134:103594. doi: 10.1016/j.micpath.2019.103594. [DOI] [PubMed] [Google Scholar]
- 50.Cappoli N, Mezzogori D, Tabolacci E, Coletta I, Navarra P, Pani G, et al. The mTOR kinase inhibitor rapamycin enhances the expression and release of pro-inflammatory cytokine interleukin 6 modulating the activation of human microglial cells. EXCLI J. 2019;18:779–798. doi: 10.17179/excli2019-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.