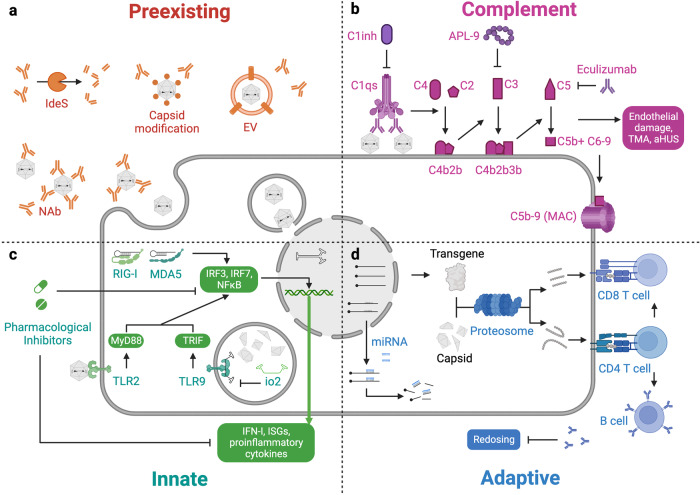
Fig. 6.
Immune responses in rAAV-based gene therapy. a Pre-existing AAV-specific antibodies can interact with rAAV and block its target cell entry. Bacteria-derived endopeptidase IdeS and its homologs can cleave the intact antibody into Fab and Fc fragments, thus reducing its half-life in circulation and eliminating Fc-mediated functions. Alternatively, the capsid can be modified or encapsulated by EVs to prevent antibody recognition. b Complement activation is observed when high doses of rAAV are administered. Though the exact mechanism remains unclear, a leading hypothesis is that rAAV adhering to the cell surface activates the classical pathway by binding to complement C1qs, which cleaves C4 and C2 to form the C3 convertase C4b2b. C3 is then cleaved and forms C5 convertase that cleaves C5 into C5b, which associates with C6–9 to form the membrane attack complex (MAC) that directly causes cell lysis, resulting in liver or kidney injury. Complement activation mediates endothelial damage, thrombotic microangiopathy (TMA), and atypical hemolytic uremic syndrome (aHUS). Cyclic peptide APL-9 is a C1 inhibitor (C1inh) and eculizumab is an inhibitor of C1, C3, and C5, which may suppress the complement activation cascade. c Innate receptors, including TLR2 for virus capsid, TLR9 for unmethylated CpG, and RIG-I/MDA5 for dsRNA, have been shown to promote inflammation. Through a series of signal transduction events, IRF3, IRF7, and NF-κB will become phosphorylated and translocate to the nucleus, where they activate type I interferons (IFN-I), interferon-stimulated genes (ISGs), and proinflammatory cytokines that mediate antiviral immunity and propagate inflammation. Many pathway components can be targeted by pharmacological inhibitors. In addition, the telomere-derived io2 sequence can suppress TLR9-mediated recognition of the transgene. d Transgene and capsid can be processed by the proteasome into peptides, which are presented by MHC-I or MHC-II molecules. CD8 and CD4 T cells that recognize those peptides will become activated. CD8 T cells may directly kill the rAAV-transduced cell, while CD4 T cells can help both CD8 T cells and B cells to enhance their effector function. B cells with specificity to the capsid can release antibodies that eliminate future possibilities of redosing. miRNA-binding sites engineered to the transgene can interact with cellular miRNA, leading to transcript degradation. In antigen-presenting cells, this design may prevent the presentation of transgene-derived peptides to T cells. In addition, anti-CD20 antibody rituximab and mTOR inhibitor rapamycin can be used to reduce adaptive responses. Figure created with Biorender.com