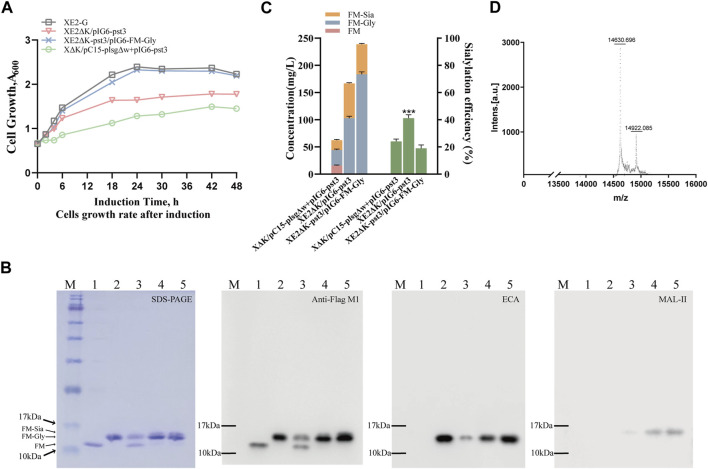
FIGURE 3.
Production of human-like sialylated N-glycoproteins in glyco-engineered E. coli. (A) Growth curves of strains expressing α-2,3-sialyltransferase pst3 in different expression modes before and after genomic integration. (B) SDS-PAGE with Coomassie brilliant blue staining, followed by immunoblotting using anti-Flag M1 antibody and lectin blotting using Gal-β-1,4-GlcNAc-specific Erythrina cristagalli lectin (ECA) and Neu5Ac-α-2,3-Gal/GalNAc-specific Maackia amurensis lectin II (MAL-II). Lanes 1-2 represent purified unmodified protein FM and glycosylated modified protein FM-Gly, while Lanes 3-5 show sialylated protein FM-Sia expressed from dual-plasmid XΔK/pC15-plsgΔw + pIG6-pst3, single plasmid XE2ΔK/pIG6-pst3 and genomic XE2ΔK-pst3/pIG6-FM-Gly, respectively. (C) Quantitative analysis of glycosylation patterns of the strains depicted in Fig. B and three independent growth curves were used to generate the stacked histograms representing the average glycosylation efficiency with the following colors: pink (FM), blue (FM-Gly), orange (FM-Sia). Green represents the average sialylation efficiency, and the modification efficiency of sialylated proteins depends on the system used. (D) MALDI-TOF detection of glycoproteins biosynthesis in strain XE2ΔK/pIG6-pst3. Data are presented as mean values +/− SD (n = 3 independent experiments). ***p < 0.001, student’s t-test.