Figure 3.
Local ABE administration restores dystrophin expression in adult humanized DMDE30mut mice
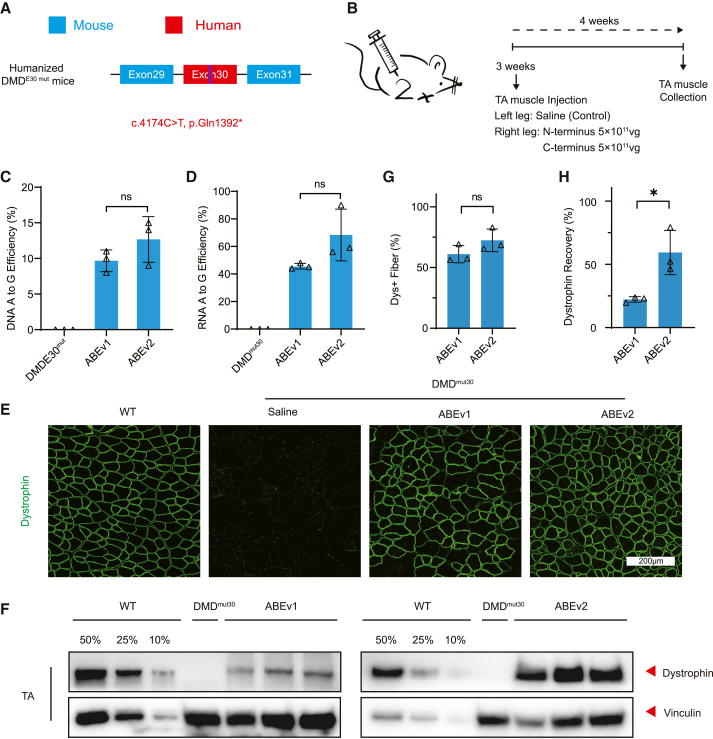
(A) Schematic for the c.4174C>T nonsense mutation in human exon 30 sequence of the humanized DMDE30mut mice. (B) Overview of the intramuscular application of split-ABE system components in 8-week-old DMDE30mut mice. The TA muscles in mouse right legs were infused with AAV-expressing N- and C-terminal ABEs at the dose of 5 × 1011 vg/leg/AAV, while the saline was injected into left legs as negative controls. All mice were dissected for analyses at 3 weeks post-injection. (C) The percentages of genomic edits in mouse TA muscles with ABE or saline treatment (n = 3). (D) Deep-sequencing of the percentage of editing events in the transcripts of mouse TA muscles with ABE or saline treatment (n = 3). (E) Immunofluorescence analysis of dystrophin and spectrin expressions in the TA muscles of age-matched WT mice and DMDE30mut mice with ABE or saline treatment. Dystrophin is shown in green. Scale bar, 200 μm. (F) Western blot analysis of dystrophin and vinculin proteins in mouse TA muscles with ABE or saline treatment. The proteins from age-matched WT mice were used to standardize dystrophin expression levels. (G) Quantification of dystrophin+ myofibers in the cross-sections of mouse TA muscles as in (E). (H) Quantification of dystrophin expression level in the TA muscles from treated DMDE30mut mice shown as in (F). Quantitative data are calculated after normalization to the vinculin expression. Quantification is shown as mean ± SEM (n = 3). Each triangle represents an individual mouse. p value was evaluated with Student t test. NS, not significant; ∗p < 0.05.