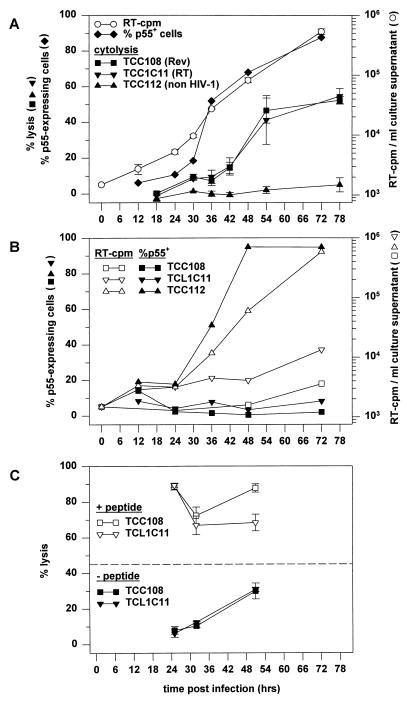
FIG. 3.
Kinetics of HIV-1 production and lysis of infected cells by Rev- and RT-specific CTL. (A) CD4+ TCL2H7 cells were infected as described in Materials and Methods, and culture supernatants were harvested at the indicated times for analysis of virus production. The TCL2H7 cells were analyzed for p55 expression and for susceptibility to CTL-mediated lysis by Rev-specific clone TCC108, RT-specific clone TCL1C11, and non-HIV-specific clone TCC112. The effector-to-target ratios were 10:1. Lysis of uninfected CD4+ TCL2H7 cells was below 5% in all assays (data not shown). The chromium release data are plotted as the average (with standard error) for triplicates at the time point at which the chromium release assay was terminated, i.e., 6 hours after the addition of chromium. This time was required for the chromium labelling (1 h), washing of the target cells and preparing the cocultures of the effector and target cells (1 h), and incubation (4 h). (B) p55 expression (closed symbols) and virus production (open symbols) by TCL2H7 cells in the presence of TCC108 cells, TCL1C11 cells, or TCC112 cells. Effector and target cells were discriminated by flow cytometric analyses of CD8 and CD4 expression, respectively. The population of p55-expressing TCL2H7 cells is expressed as a percentage of the CD8− cells and not of the CD4+ cells, since CD4 was down-regulated in a major fraction of the infected cells. (C) Infected TCL2H7 cells were analyzed in chromium release assays after incubation without peptide or with the relevant peptides at 10 μM: SAEPVPLQL for TCC108 cells and IVLPEKDSW for TCL1C11 cells. The average specific lysis (with standard error) for triplicates is shown. The dashed line shows the percentage of p55-expressing cells at 48 h after infection. Lysis of uninfected TCL2H7 cells without peptides was always below 5% (data not shown), and peptide-pulsed uninfected TCL2H7 cells were lysed as efficiently as peptide-pulsed infected cells (data not shown).