Abstract
Hepatic artery thrombosis (HAT) is a devastating vascular complication following liver transplantation, requiring prompt diagnosis and rapid revascularization treatment to prevent graft loss. At present, imaging modalities such as ultrasound, computed tomography, and magnetic resonance play crucial roles in diagnosing HAT. Although imaging techniques have improved sensitivity and specificity for HAT diagnosis, they have limitations that hinder the timely diagnosis of this complication. In this sense, the emergence of artificial intelligence (AI) presents a transformative opportunity to address these diagnostic limitations. The develo pment of machine learning algorithms and deep neural networks has demon strated the potential to enhance the precision diagnosis of liver transplant com plications, enabling quicker and more accurate detection of HAT. This article examines the current landscape of imaging diagnostic techniques for HAT and explores the emerging role of AI in addressing future challenges in the diagnosis of HAT after liver transplant.
Keywords: Liver transplantation, Postoperative complications, Hepatic artery, Throm bosis, Radiology, Artificial intelligence
Core Tip: Hepatic artery thrombosis (HAT) is a severe vascular complication after liver transplant requiring prompt diagnosis and intervention to prevent graft loss and patient death. However, current imaging methods have limitations. Artificial intelligence (AI), especially deep learning, holds promising potential to enhance precise and accurate HAT diagnosis. This article explores current HAT imaging techniques and highlights the potential role of AI-based methods, aiming to improve diagnostic performance and recipient survival.
INTRODUCTION
Liver transplantation has emerged as the treatment of choice for patients with end-stage liver diseases (ESLD), including advanced stages of both cholestatic and non-cholestatic cirrhosis, as well as the early stages of hepatocellular carcinoma[1-3]. In recent years, there has been a sustained increase in liver transplant cases, resulting in improved survival pro gnoses and quality of life for ESLD patients[4,5].
Continuous progress in the development of surgical techniques and novel immunosuppressive agents has contributed to enhanced survival rates among recipients[6,7], with a current five-year survival rate of up to 75%[8-10]. However, strict postoperative multidisciplinary surveillance is imperative to identify and address potential complications that may affect both the graft and the recipient[11,12]. Despite ongoing advancements in the field of liver transplantation, postoperative vascular complications, particularly those related to the hepatic artery (HA), remain one of the primary causes of graft failure and recipient mortality[11].
HA thrombosis (HAT) is a severe complication after liver transplantation, associated with biliary complications such as ischemic cholangiopathy, which may occur even after a successful revascularization treatment, resulting in late graft loss and therefore having a critical impact on quality of life[12,13]. Furthermore, HAT is considered as a risk factor for deve lopment of biliary stones in liver graft, which is associated with recurring cholangitis, secondary biliary cirrhosis, and graft failure[14,15].
HAT can be classified according to its temporal onset. Thrombotic occlusion of the HA occurring within the first 30 d following liver transplantation is classified as early HAT (eHAT), which is believed to result from technical problems and perioperative risk factors such as artery kinking, donor arterial anatomic variation, different diameters of the arteries in the anastomosis, or low quality of the donor’s or recipient’s arteries[16,17].
On the other hand, the later development of HAT, known as late HAT, is usually related to ischemic or immunologic risk factors such as cytomegalovirus-positive donors and hepatitis C seropositive recipient[17-19]. A large study including 4234 cases of adult and pediatric liver transplants reported an overall HAT incidence of 5%, which was higher in pediatric liver transplant recipients than in adults (8% vs 3.9% respectively)[20]. In addition, a systematic review comprising 21822 cases of orthotopic liver transplantation, reported an overall incidence of eHAT of 4.4% with an overall mortality of 33.3%, which was also significantly higher in children (34.3%) than in adults (25%)[16].
Strikingly, the cause of this difference remains unknown. Nevertheless, the most likely explanation is the small size of the vessels and the associated technical difficulties of anastomosing[16,21,22]. The reported incidence of late HAT is highly variable, ranging widely from 1% to 25%, with mortality rates of 50%[20,23]. In addition, median times reported to diagnosis of eHAT were 6.9 d (range: 1-17.5 d postoperative), while for late HAT, median times were 6 mo, ranging from 1.8 to 79 mo[16].
The clinical presentation of HAT widely varies according to the timing of onset and the development of collateral vessels, which could maintain blood flow to the allograft[17,20]. Clinically, eHAT manifests with fever, abdominal pain, elevated transaminases, and leukocytosis, which can be followed by septic shock[20,24-27]. Late HAT has an insidious course, characterized by progressive abdominal pain, alteration of liver function tests, relapsing fever, recurring cholangitis, and bacteremia[27,28].
Color-doppler ultrasound (CDUS) is the modality of choice for the postoperative surveillance of liver graft vasculature during the postoperative period, which could depict hemodynamic changes that require further assessment with second-line diagnostic tools such as computed tomography angiography (CTA) or conventional hepatic arterial angiography[17,29,30].
Currently, there are three different modalities for HAT treatment: Retransplantation, surgical revascularization, and endovascular revascularization[17,25]. However, the most effective treatment approach remains controversial[24]. Bekker et al[16], reported that retransplantation was more frequently performed in pediatric liver transplant recipients (61.9%) than in adults (50%), and was the treatment of choice in the overall cases of eHAT. In another large study, retransplantation was performed in 71% of patients with eHAT and 51% of patients with late HAT[20].
CTA is the second line of choice when a hemodynamic HA abnormality is suspected on doppler ultrasound evaluation[29,31]. The interpretation of CTA still requires a detailed evaluation of all abdominal vascular structures, which is a time- and labor-intensive process that requires high expertise in abdominal imaging. Although several studies have reported the high sensitivity of CTA for HAT diagnosis, its specificity remains somewhat low (83.5-87.5%)[32,33].
In this sense, considering the invasiveness and risk of diagnostic angiography, which is the current gold standard for HAT confirmation, it is necessary to improve the diagnostic performance of CTA[34]. The emergence of artificial intel ligence (AI), particularly deep learning (DL) algorithms, is gaining growing attention for its performance in image-recognition tasks, achieving high performance on CTA analysis[35-37].
Recent studies have developed different DL-based algorithms, which have resulted in shorter time and high diagnostic performance for CTA diagnosis of vessel occlusion at different anatomic sites, thus improving management outcomes of vascular time-dependent pathologies[37-41].
This article explores the current landscape of multimodality imaging for HAT, highlighting the potential of DL-based algorithms as emerging technologies that could improve HAT diagnosis post-liver transplantation.
MULTIMODALITY IMAGING OF HEPATIC ARTERY THROMBOSIS AFTER LIVER TRANSPLANTATION
Ultrasound (US) evaluation is the modality of choice for assessing liver graft vasculature. It offers a rapid, comprehensive, and accurate grayscale and CDUS evaluation of liver parenchyma at the patient's bedside[29,42], which allows precise assessment of the entire graft vasculature, particularly the blood flow in the HA anastomosis[28].
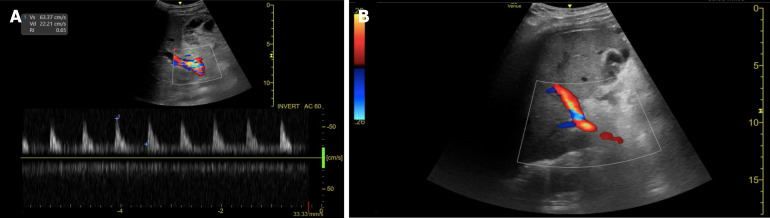
The arterial anastomosis is typically located in the porta hepatis and can be identified by the presence of intense focal color aliasing and elevated velocity on spectral doppler images surrounding the porta hepatis[43]. Normal doppler evaluation of the HA shows a continuous diastolic flow with a rapid systolic upstroke, an acceleration time of less than 80 msec, and a resistive index that ranges between 0.5 and 0.7[44] (Figure 1).
Figure 1.
Doppler ultrasound evaluation of the hepatic artery. A: Intercostal color and spectral doppler image of a normal hepatic artery at the porta hepatis in the liver graft of a 51-year-old woman on postoperative day 3 after transplant, depicting a rapid systolic upstroke with continuous low-velocity diastolic flow and a normal resistive index; B: Subcostal color doppler image of the right hepatic lobe in a 46-year-old man on postoperative day 2 demonstrates vascular flow in the portal vein, with no hepatic artery flow detected on color or spectral doppler images at the porta hepatis.
In 1996, Nolten and Sproat[45] described some qualitative hemodynamic changes in the HA that may anticipate its thrombotic occlusion, including the loss of diastolic flow, dampening of the systolic peak, and finally, the complete loss of arterial flow. The detection of low-velocity and high-resistance flow, nonvisualization, or absence of Doppler color flow in the HA and its intrahepatic branches are findings highly suggestive of HAT[24,46], requiring prompt assessment using CTA or conventional hepatic angiography[44,47].
CTA plays a crucial role in the detection of HAT following liver transplantation. Its high-resolution, contrast-enhanced images provide detailed anatomical information, making it a crucial tool for diagnosing HAT, allowing the assessment of vessel patency and identification of thrombus formation, as well as the evaluation of collateral circulation and ischemia-related biliary complications such as biloma and abscess[48,49].
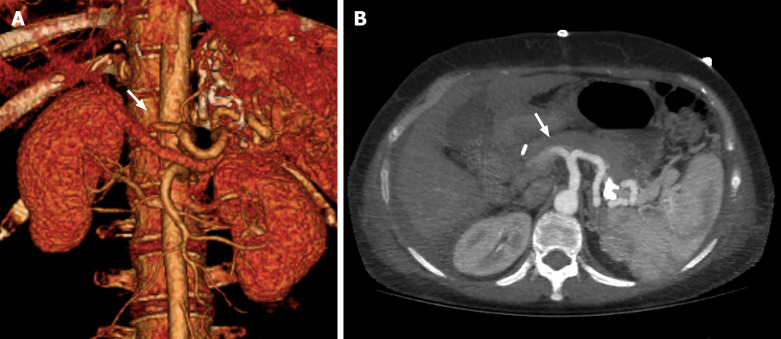
The lack of opacification of HA and its intrahepatic branches strongly suggest eHAT. However, it should be confirmed in specific detail with maximum intensity projection images[25,48] (Figure 2). On the other hand, the development of collaterals, mainly raised from the phrenic arteries, and the temporal onset are crucial signs that suggest the diagnosis of late HAT[11,16].
Figure 2.
Computed tomography angiography evaluation of hepatic artery thrombosis in liver graft. A 51-year-old woman on postoperative day 7 after a liver transplant. A: Axial abdominal computed tomography angiography (CTA) images at maximum intensity projection (white arrow); B: Coronal 3D volume rendering CTA reconstruction showing absence of vascular opacification of vessels distal to occlusion of the hepatic artery (white arrow).
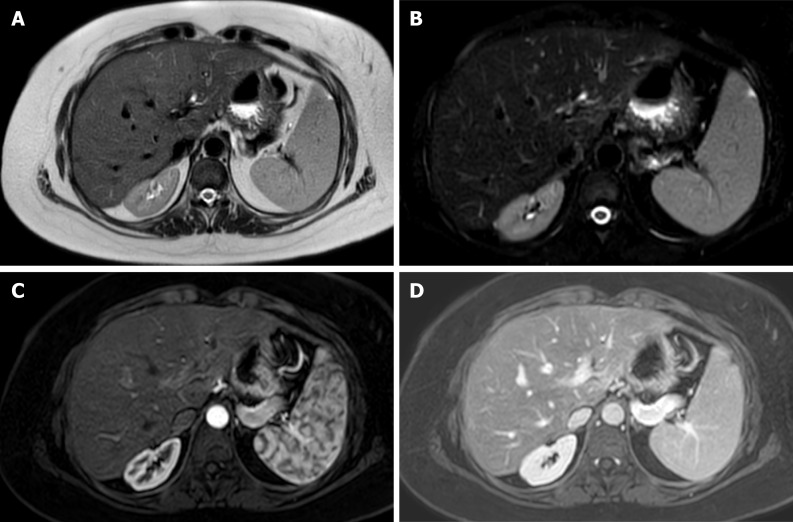
Magnetic resonance offers detailed images of the graft parenchyma and biliary ducts within the postoperative surveillance period (Figure 3). However, it may be less readily available and time-consuming compared to the US and CTA[50]. In addition, retrospective studies have reported similar diagnostic accuracy to US but with a higher number of false positives and a more demanding examination[51].
Figure 3.
Magnetic resonance imaging of liver graft. Axial T2-weighted single-shot fast spin-echo image. A: Fat saturation (Fat-sat); B: Contrast enhanced T1-weighted gradient-echo image in late arterial; C: Portal phase; D: Depicts a homogeneous signal intensity at graft parenchyma, with adequate representation of intrahepatic and extrahepatic arterial branches, venous vessels and biliary ducts, in a 32-year-old man on postoperative surveillance after a liver transplantation.
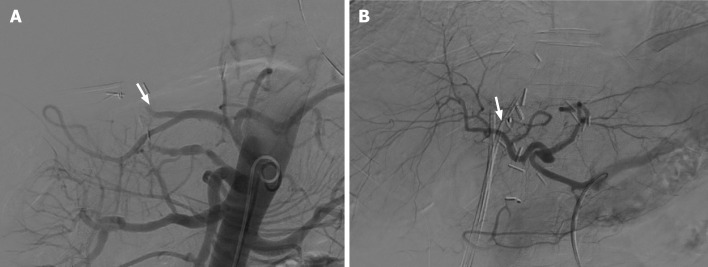
As mentioned above, hepatic arterial angiography is considered the gold standard for the diagnosis of HAT, which can involve diagnostic and therapeutic approaches for the endovascular management of this complication[52] (Figure 4).
Figure 4.
Visceral angiography performed 3 d after orthotopic liver transplant. A: Demonstrated complete occlusion of the hepatic artery (white arrow); B: Recanalization of the hepatic artery after thrombectomy, with improved intrahepatic blood flow (white arrow).
EMERGING ROLE OF DL IN HEPATIC ARTERY THROMBOSIS DIAGNOSIS
AI has emerged as a revolutionary technology with a critical impact on the field of medicine. By enhancing diagnostic accuracy, improving efficiency, and enabling early detection of diseases, its continued integration into radiology practices holds the promise of further improving patient care and advancing our understanding of complex diseases[53,54].
Recent research showed that AI-based technology can significantly support the field of liver transplantation by optimizing organ allocation, donor-recipient matching, survival prediction analysis, and the diagnosis of postoperative complications in liver graft recipients[55,56].
As mentioned above, AI algorithms have improved the analysis of medical images, detecting subtle abnormalities, quantifying disease progression, and identifying patterns that might be challenging for human radiologists to discern[57].
DL is a subfield of AI based on neural networks inspired by the human brain structure. It focuses on using artificial neural networks with multiple layers, often referred to as deep neural networks, to model and solve complex tasks and approximate very complex nonlinear relationships[57,58].
Convolutional neural networks (CNNs) are a type of DL artificial neural network specifically designed for processing and analyzing visual data, such as images and videos, for tasks involving visual perception. Therefore, the emerging CNN algorithms have had a profound impact on the field of radiology, revolutionizing the way medical images are interpreted, analyzed, and utilized for diagnosis and treatment planning[59].
Currently, the increasing use of CNN algorithms in medical image analysis has demonstrated interesting results in improving rapid frontline CTA detection of life-threatening large vessel occlusion, with promising diagnostic per formance[60-64].
Tajbakhsh et al[65] investigated the feasibility of a novel CNN algorithm as an emergent mechanism to improve the diagnosis of thromboembolism detection, showing that their DL algorithm outperforms classic machine learning techniques with a sensitivity of 83% for detecting thromboembolism on CTA[65,66]. Additionally, they also developed a novel computer-aided embolism diagnosis system, providing radiologists with an effective visualization tool to conveniently examine the vessel lumen from multiple perspectives and confidently report filling defects. Their vessel-oriented image representation offers a multi-view representation of the embolus, summarizing the 3D contextual information around it[67].
Huan et al[68] developed the PENet-3D CNN model to detect thromboembolic occlusion using the entire volumetric CTA imaging data, achieving an areas under receiver operating characteristic curve (AUROC) of 0.85. Later, they optimize their model by integrating clinical data from the electronic medical record to achieve 0.87 [95%CI: 0.871-0.875], 0.87 [95%CI: 0.872-0.877], and 0.947 [95%CI: 0.946-0.948] of sensitivity, specificity, and AUROC respectively, for the task of automatically detecting thromboembolism on volumetric CTA image analysis[69].
Ma et al[70] proposed a new DL model for embolism detection using the CNN-based network Gradient-weighted Class Activation Mapping (Grad-Cam), a localization technique that provides visual explanations on CTA scans. The algorithm achieved a sensitivity of 0.86 with a specificity of 0.85, which is competitive with radiologists' sensitivities ranging from 0.67 to 0.87 and specificities of 0.89-0.99 for embolism detection on CTA[71].
A recent multicenter study was performed to validate a DL-based application designed with CNN (CINA-PE), to automatically detect embolism on CTA and alert radiologists for urgent interpretation. This algorithm achieved a sensitivity of 91.4% (95%CI: 86.4%-95.0%) and specificity of 91.5% (95%CI: 86.8%-95.0%), leading to an accuracy of 91.5%[72].
Additionally, an automated CNN-based algorithm designed by Fu et al[39], that could be trained to complete lumen segmentation automatically reduced the radiologists report writing time of CTA from 28.8 min ± 5.6 to 12.4 min ± 2.0. Therefore, it offers a time-saving and accurate method to analyze CTA to provide optimized clinical workflow.
These experiments further confirm the potential of DL algorithms for medical imaging applications[59]. In particular, the implementation of CNN-based algorithms for image analysis in patients with high clinical suspicion of thrombotic occlusion of HA within the perioperative period could improve the diagnostic performance of the radiologist, optimizing its sensitivity, specificity, and report writing time. Thereby leading to an early and efficient multidisciplinary workflow and therapeutic response, ultimately improving patient prognosis.
CONCLUSION
Despite the continuous advances in the field of liver transplantation, HAT remains a significant cause of morbidity and mortality in recipient patients. While there are different imaging studies that allow the assessment of the HA, they have limitations that prevent an early diagnosis of this complication.
AI can potentially revolutionize HAT detection by enhancing the interpretation of imaging data and facilitating rapid and precise diagnosis. The integration of AI into existing imaging modalities, such as CTA, holds the potential to streamline clinical workflows, reduce healthcare costs, and ultimately improve patient outcomes.
Future investigations should be focused on improving the diagnostic performance of non-invasive imaging techniques for life-threating diseases. HAT is a severe complication that significantly increases the risk of graft loss and patient mortality. In this regard, emergent DL-based algorithms have demonstrated high diagnostic performance for arterial occlusion at different anatomical sites. Considering these findings, the development of new DL algorithms focused on the CTA analysis of the liver graft vasculature could assist radiologists in improving sensitivity, specificity, and diagnostic reporting time for HAT, thus enhancing early treatment for this time-dependent complication.
Footnotes
Conflict-of-interest statement: The authors declare that there is no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 16, 2023
First decision: November 23, 2023
Article in press: December 29, 2023
Specialty type: Transplantation
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Mogahed EA, Egypt; Mucenic M, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
Contributor Information
Cristian Lindner, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile; Department of Radiology, Hospital Clínico Regional Guillermo Grant Benavente, Concepción 4030000, Chile. clindner@udec.cl.
Raúl Riquelme, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile; Department of Radiology, Hospital Clínico Regional Guillermo Grant Benavente, Concepción 4030000, Chile.
Rodrigo San Martín, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile; Department of Radiology, Hospital Clínico Regional Guillermo Grant Benavente, Concepción 4030000, Chile.
Frank Quezada, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile; Department of Radiology, Hospital Clínico Regional Guillermo Grant Benavente, Concepción 4030000, Chile.
Jorge Valenzuela, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile; Department of Radiology, Hospital Clínico Regional Guillermo Grant Benavente, Concepción 4030000, Chile.
Juan P Maureira, Department of Statistics, Catholic University of Maule, Talca 3460000, Chile.
Martín Einersen, Department of Radiology, Faculty of Medicine, University of Concepción, Concepción 4030000, Chile; Neurovascular Unit, Department of Radiology, Hospital Clínico Regional Guillermo Grant Benavente, Concepción 4030000, Chile.
References
- 1.Fatima I, Jahagirdar V, Kulkarni AV, Reddy R, Sharma M, Menon B, Reddy DN, Rao PN. Liver Transplantation: Protocol for Recipient Selection, Evaluation, and Assessment. J Clin Exp Hepatol. 2023;13:841–853. doi: 10.1016/j.jceh.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadlowiec CC, Taner T. Liver transplantation: Current status and challenges. World J Gastroenterol. 2016;22:4438–4445. doi: 10.3748/wjg.v22.i18.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 4.Chu KK, Wong KH, Chok KS. Expanding Indications for Liver Transplant: Tumor and Patient Factors. Gut Liver. 2021;15:19–30. doi: 10.5009/gnl19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodzin AS, Baker TB. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transpl. 2018;24:1470–1475. doi: 10.1002/lt.25320. [DOI] [PubMed] [Google Scholar]
- 6.Baheti AD, Sanyal R, Heller MT, Bhargava P. Surgical Techniques and Imaging Complications of Liver Transplant. Radiol Clin North Am. 2016;54:199–215. doi: 10.1016/j.rcl.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Charlton M, Levitsky J, Aqel B, OʼGrady J, Hemibach J, Rinella M, Fung J, Ghabril M, Thomason R, Burra P, Little EC, Berenguer M, Shaked A, Trotter J, Roberts J, Rodriguez-Davalos M, Rela M, Pomfret E, Heyrend C, Gallegos-Orozco J, Saliba F. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation. 2018;102:727–743. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 8.Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, Pageaux GP, Radenne S, Trebicka J, Fernandez J, Perricone G, Piano S, Nadalin S, Morelli MC, Martini S, Polak WG, Zieniewicz K, Toso C, Berenguer M, Iegri C, Invernizzi F, Volpes R, Karam V, Adam R, Faitot F, Rabinovich L, Saliba F, Meunier L, Lesurtel M, Uschner FE, Fondevila C, Michard B, Coilly A, Meszaros M, Poinsot D, Schnitzbauer A, De Carlis LG, Fumagalli R, Angeli P, Arroyo V, Jalan R ELITA/EF-CLIF working group. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS) J Hepatol. 2021;75:610–622. doi: 10.1016/j.jhep.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 9.Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, Schnellinger EM, Handarova D, Weiss S, Cafarella M, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2021 Annual Data Report: Liver. Am J Transplant. 2023;23:S178–S263. doi: 10.1016/j.ajt.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136–1142. doi: 10.1097/TP.0000000000003174. [DOI] [PubMed] [Google Scholar]
- 11.Bastón Castiñeiras M, Benítez Linero I, Serrano Zarcero V, Fernández Castellano G, Suárez-Artacho G, López Romero JL. Hepatic Artery Thrombosis After Orthotopic Liver Transplant: Experience in the Last 10 Years. Transplant Proc. 2022;54:51–53. doi: 10.1016/j.transproceed.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Fujiki M, Hashimoto K, Palaios E, Quintini C, Aucejo FN, Uso TD, Eghtesad B, Miller CM. Probability, management, and long-term outcomes of biliary complications after hepatic artery thrombosis in liver transplant recipients. Surgery. 2017;162:1101–1111. doi: 10.1016/j.surg.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Guirguis RN, Nashaat EH, Yassin AE, Ibrahim WA, Saleh SA, Bahaa M, El-Meteini M, Fathy M, Dabbous HM, Montasser IF, Salah M, Mohamed GA. Impact of biliary complications on quality of life in live-donor liver transplant recipients. World J Hepatol. 2021;13:1405–1416. doi: 10.4254/wjh.v13.i10.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabbous H, Elsayed A, Salah M, Montasser I, Atef M, Elmetenini M. Risk factors and management of biliary stones after living donor liver transplant and its effect on graft outcome. Front Med (Lausanne) 2022;9:927744. doi: 10.3389/fmed.2022.927744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kırnap M, Ayvazoğlu Soy EH, Akdur A, Yıldırım S, Harman A, Moray G, Haberal M. Incidence and Treatment of Bile Stones After Liver Transplant. Exp Clin Transplant. 2017 doi: 10.6002/ect.2017.0023. [DOI] [PubMed] [Google Scholar]
- 16.Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009;9:746–757. doi: 10.1111/j.1600-6143.2008.02541.x. [DOI] [PubMed] [Google Scholar]
- 17.Piardi T, Lhuaire M, Bruno O, Memeo R, Pessaux P, Kianmanesh R, Sommacale D. Vascular complications following liver transplantation: A literature review of advances in 2015. World J Hepatol. 2016;8:36–57. doi: 10.4254/wjh.v8.i1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutluturk K, Sahin TT, Karakas S, Unal B, Gozukara Bag HG, Akbulut S, Aydin C, Yilmaz S. Early Hepatic Artery Thrombosis After Pediatric Living Donor Liver Transplantation. Transplant Proc. 2019;51:1162–1168. doi: 10.1016/j.transproceed.2019.01.104. [DOI] [PubMed] [Google Scholar]
- 19.Puliti Reigada CH, de Ataide EC, de Almeida Prado Mattosinho T, Boin IFSF. Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas. Transplant Proc. 2017;49:867–870. doi: 10.1016/j.transproceed.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg. 2009;208:896–903; discussion 903. doi: 10.1016/j.jamcollsurg.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Shirouzu Y, Kasahara M, Morioka D, Sakamoto S, Taira K, Uryuhara K, Ogawa K, Takada Y, Egawa H, Tanaka K. Vascular reconstruction and complications in living donor liver transplantation in infants weighing less than 6 kilograms: the Kyoto experience. Liver Transpl. 2006;12:1224–1232. doi: 10.1002/lt.20800. [DOI] [PubMed] [Google Scholar]
- 22.Mori K, Nagata I, Yamagata S, Sasaki H, Nishizawa F, Takada Y, Moriyasu F, Tanaka K, Yamaoka Y, Kumada K. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation--its surgical advantages compared with conventional procedures. Transplantation. 1992;54:263–268. doi: 10.1097/00007890-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Gunsar F, Rolando N, Pastacaldi S, Patch D, Raimondo ML, Davidson B, Rolles K, Burroughs AK. Late hepatic artery thrombosis after orthotopic liver transplantation. Liver Transpl. 2003;9:605–611. doi: 10.1053/jlts.2003.50057. [DOI] [PubMed] [Google Scholar]
- 24.Singhal A, Stokes K, Sebastian A, Wright HI, Kohli V. Endovascular treatment of hepatic artery thrombosis following liver transplantation. Transpl Int. 2010;23:245–256. doi: 10.1111/j.1432-2277.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- 25.Pareja E, Cortes M, Navarro R, Sanjuan F, López R, Mir J. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc. 2010;42:2970–2972. doi: 10.1016/j.transproceed.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 26.Drazan K, Shaked A, Olthoff KM, Imagawa D, Jurim O, Kiai K, Shackelton C, Busuttil R. Etiology and management of symptomatic adult hepatic artery thrombosis after orthotopic liver transplantation (OLT) Am Surg. 1996;62:237–240. [PubMed] [Google Scholar]
- 27.Silva MA, Jambulingam PS, Gunson BK, Mayer D, Buckels JA, Mirza DF, Bramhall SR. Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl. 2006;12:146–151. doi: 10.1002/lt.20566. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharjya S, Gunson BK, Mirza DF, Mayer DA, Buckels JA, McMaster P, Neuberger JM. Delayed hepatic artery thrombosis in adult orthotopic liver transplantation-a 12-year experience. Transplantation. 2001;71:1592–1596. doi: 10.1097/00007890-200106150-00018. [DOI] [PubMed] [Google Scholar]
- 29.Brookmeyer CE, Bhatt S, Fishman EK, Sheth S. Multimodality Imaging after Liver Transplant: Top 10 Important Complications. Radiographics. 2022;42:702–721. doi: 10.1148/rg.210108. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Qian S, Liu R, Yuan W, Wang JH. Interventional Treatment for Hepatic Artery Thrombosis after Liver Transplantation. J Vasc Interv Radiol. 2017;28:1116–1122. doi: 10.1016/j.jvir.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093–1114. doi: 10.1148/rg.235035031. [DOI] [PubMed] [Google Scholar]
- 32.Kayahan Ulu EM, Coskun M, Ozbek O, Tutar NU, Ozturk A, Aytekin C, Haberal M. Accuracy of multidetector computed tomographic angiography for detecting hepatic artery complications after liver transplantation. Transplant Proc. 2007;39:3239–3244. doi: 10.1016/j.transproceed.2007.08.097. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Kim KW, Lee J, Kwon HJ, Kwon JH, Song GW, Lee SG. Diagnostic Performance for Hepatic Artery Occlusion After Liver Transplantation: Computed Tomography Angiography Versus Contrast-Enhanced Ultrasound. Liver Transpl. 2019;25:1651–1660. doi: 10.1002/lt.25588. [DOI] [PubMed] [Google Scholar]
- 34.Kim JS, Kim DW, Kim KW, Song GW, Lee SG. Improving the Specificity of CT Angiography for the Diagnosis of Hepatic Artery Occlusion after Liver Transplantation in Suspected Patients with Doppler Ultrasound Abnormalities. Korean J Radiol. 2022;23:52–59. doi: 10.3348/kjr.2021.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao J, Huang L, Qu M, Chen B, Wang G. Artificial Intelligence in Coronary CT Angiography: Current Status and Future Prospects. Front Cardiovasc Med. 2022;9:896366. doi: 10.3389/fcvm.2022.896366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Mao J, Sun N, Yu X, Chai L, Tian Y, Wang J, Liang J, Tao H, Yuan L, Lu J, Wang Y, Zhang B, Wu K, Chen M, Wang Z, Lu L. Deep Learning for Detection of Intracranial Aneurysms from Computed Tomography Angiography Images. J Digit Imaging. 2023;36:114–123. doi: 10.1007/s10278-022-00698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, Ran AR, Nguyen TX, Lin TPH, Chen H, Lai TYY, Tham CC, Cheung CY. Deep Learning in Optical Coherence Tomography Angiography: Current Progress, Challenges, and Future Directions. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu F, Shan Y, Yang G, Zheng C, Zhang M, Rong D, Wang X, Lu J. Deep Learning for Head and Neck CT Angiography: Stenosis and Plaque Classification. Radiology. 2023;307:e220996. doi: 10.1148/radiol.220996. [DOI] [PubMed] [Google Scholar]
- 40.Hwang JH, Seo JW, Kim JH, Park S, Kim YJ, Kim KG. Comparison between Deep Learning and Conventional Machine Learning in Classifying Iliofemoral Deep Venous Thrombosis upon CT Venography. Diagnostics (Basel) 2022;12 doi: 10.3390/diagnostics12020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai L, Zhou Q, Zhou H, Zhang H, Cheng P, Ding M, Xu X, Zhang X. Deep learning-based classification of lower extremity arterial stenosis in computed tomography angiography. Eur J Radiol. 2021;136:109528. doi: 10.1016/j.ejrad.2021.109528. [DOI] [PubMed] [Google Scholar]
- 42.Maheshwari E, Tublin ME. Sonography of liver transplantation. Abdom Radiol (NY) 2021;46:68–83. doi: 10.1007/s00261-020-02799-7. [DOI] [PubMed] [Google Scholar]
- 43.Craig EV, Heller MT. Complications of liver transplant. Abdom Radiol (NY) 2021;46:43–67. doi: 10.1007/s00261-019-02340-5. [DOI] [PubMed] [Google Scholar]
- 44.Di Martino M, Rossi M, Mennini G, Melandro F, Anzidei M, De Vizio S, Koryukova K, Catalano C. Imaging follow-up after liver transplantation. Br J Radiol. 2016;89:20151025. doi: 10.1259/bjr.20151025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolten A, Sproat IA. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198:553–559. doi: 10.1148/radiology.198.2.8596865. [DOI] [PubMed] [Google Scholar]
- 46.Flint EW, Sumkin JH, Zajko AB, Bowen A. Duplex sonography of hepatic artery thrombosis after liver transplantation. AJR Am J Roentgenol. 1988;151:481–483. doi: 10.2214/ajr.151.3.481. [DOI] [PubMed] [Google Scholar]
- 47.Uzochukwu LN, Bluth EI, Smetherman DH, Troxclair LA, Loss GE Jr, Cohen A, Eason JD. Early postoperative hepatic sonography as a predictor of vascular and biliary complications in adult orthotopic liver transplant patients. AJR Am J Roentgenol. 2005;185:1558–1570. doi: 10.2214/AJR.04.1258. [DOI] [PubMed] [Google Scholar]
- 48.Kim SY, Kim KW, Kim MJ, Shin YM, Lee MG, Lee SG. Multidetector row CT of various hepatic artery complications after living donor liver transplantation. Abdom Imaging. 2007;32:635–643. doi: 10.1007/s00261-006-9145-5. [DOI] [PubMed] [Google Scholar]
- 49.Delgado-Moraleda JJ, Ballester-Vallés C, Marti-Bonmati L. Role of imaging in the evaluation of vascular complications after liver transplantation. Insights Imaging. 2019;10:78. doi: 10.1186/s13244-019-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao CC, Chen MH, Yu CY, Tsang LL, Chen CL, Hsu HW, Lim WX, Chuang YH, Huang PH, Cheng YF, Ou HY. Non-Contrast-Enhanced and Contrast-Enhanced Magnetic Resonance Angiography in Living Donor Liver Vascular Anatomy. Diagnostics (Basel) 2022;12 doi: 10.3390/diagnostics12020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glockner JF, Forauer AR, Solomon H, Varma CR, Perman WH. Three-dimensional gadolinium-enhanced MR angiography of vascular complications after liver transplantation. AJR Am J Roentgenol. 2000;174:1447–1453. doi: 10.2214/ajr.174.5.1741447. [DOI] [PubMed] [Google Scholar]
- 52.Abad J, Hidalgo EG, Cantarero JM, Parga G, Fernandez R, Gomez M, Colina F, Moreno E. Hepatic artery anastomotic stenosis after transplantation: treatment with percutaneous transluminal angioplasty. Radiology. 1989;171:661–662. doi: 10.1148/radiology.171.3.2524086. [DOI] [PubMed] [Google Scholar]
- 53.Haug CJ, Drazen JM. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N Engl J Med. 2023;388:1201–1208. doi: 10.1056/NEJMra2302038. [DOI] [PubMed] [Google Scholar]
- 54.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 55.Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216–1233. doi: 10.1016/j.jhep.2023.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Khorsandi SE, Hardgrave HJ, Osborn T, Klutts G, Nigh J, Spencer-Cole RT, Kakos CD, Anastasiou I, Mavros MN, Giorgakis E. Artificial Intelligence in Liver Transplantation. Transplant Proc. 2021;53:2939–2944. doi: 10.1016/j.transproceed.2021.09.045. [DOI] [PubMed] [Google Scholar]
- 57.Gore JC. Artificial intelligence in medical imaging. Magn Reson Imaging. 2020;68:A1–A4. doi: 10.1016/j.mri.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Erickson BJ. Basic Artificial Intelligence Techniques: Machine Learning and Deep Learning. Radiol Clin North Am. 2021;59:933–940. doi: 10.1016/j.rcl.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A. Deep Learning: A Primer for Radiologists. Radiographics. 2017;37:2113–2131. doi: 10.1148/rg.2017170077. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Zhou M, Ding Y, Li X, Zhou Z, Xie T, Shi Z, Fu W. Fully automatic segmentation of abdominal aortic thrombus in pre-operative CTA images using deep convolutional neural networks. Technol Health Care. 2022;30:1257–1266. doi: 10.3233/THC-THC213630. [DOI] [PubMed] [Google Scholar]
- 61.Remedios LW, Lingam S, Remedios SW, Gao R, Clark SW, Davis LT, Landman BA. Comparison of convolutional neural networks for detecting large vessel occlusion on computed tomography angiography. Med Phys. 2021;48:6060–6068. doi: 10.1002/mp.15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stib MT, Vasquez J, Dong MP, Kim YH, Subzwari SS, Triedman HJ, Wang A, Wang HC, Yao AD, Jayaraman M, Boxerman JL, Eickhoff C, Cetintemel U, Baird GL, McTaggart RA. Detecting Large Vessel Occlusion at Multiphase CT Angiography by Using a Deep Convolutional Neural Network. Radiology. 2020;297:640–649. doi: 10.1148/radiol.2020200334. [DOI] [PubMed] [Google Scholar]
- 63.Murray NM, Unberath M, Hager GD, Hui FK. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J Neurointerv Surg. 2020;12:156–164. doi: 10.1136/neurintsurg-2019-015135. [DOI] [PubMed] [Google Scholar]
- 64.Soffer S, Klang E, Shimon O, Barash Y, Cahan N, Greenspana H, Konen E. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: a systematic review and meta-analysis. Sci Rep. 2021;11:15814. doi: 10.1038/s41598-021-95249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tajbakhsh N, Shin JY, Gurudu SR, Hurst RT, Kendall CB, Gotway MB, Jianming Liang. Convolutional Neural Networks for Medical Image Analysis: Full Training or Fine Tuning? IEEE Trans Med Imaging. 2016;35:1299–1312. doi: 10.1109/TMI.2016.2535302. [DOI] [PubMed] [Google Scholar]
- 66.Tajbakhsh N, Gotway MB, Liang J. Computer-Aided Pulmonary Embolism Detection Using a Novel Vessel-Aligned Multi-planar Image Representation and Convolutional Neural Networks. Springer. 2015:62–69. [Google Scholar]
- 67.Tajbakhsh N, Shin JY, Gotway MB, Liang J. Computer-aided detection and visualization of pulmonary embolism using a novel, compact, and discriminative image representation. Med Image Anal. 2019;58:101541. doi: 10.1016/j.media.2019.101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang SC, Kothari T, Banerjee I, Chute C, Ball RL, Borus N, Huang A, Patel BN, Rajpurkar P, Irvin J, Dunnmon J, Bledsoe J, Shpanskaya K, Dhaliwal A, Zamanian R, Ng AY, Lungren MP. PENet-a scalable deep-learning model for automated diagnosis of pulmonary embolism using volumetric CT imaging. NPJ Digit Med. 2020;3:61. doi: 10.1038/s41746-020-0266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang SC, Pareek A, Zamanian R, Banerjee I, Lungren MP. Multimodal fusion with deep neural networks for leveraging CT imaging and electronic health record: a case-study in pulmonary embolism detection. Sci Rep. 2020;10:22147. doi: 10.1038/s41598-020-78888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X, Ferguson EC, Jiang X, Savitz SI, Shams S. A multitask deep learning approach for pulmonary embolism detection and identification. Sci Rep. 2022;12:13087. doi: 10.1038/s41598-022-16976-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eng J, Krishnan JA, Segal JB, Bolger DT, Tamariz LJ, Streiff MB, Jenckes MW, Bass EB. Accuracy of CT in the diagnosis of pulmonary embolism: a systematic literature review. AJR Am J Roentgenol. 2004;183:1819–1827. doi: 10.2214/ajr.183.6.01831819. [DOI] [PubMed] [Google Scholar]
- 72.Grenier PA, Ayobi A, Quenet S, Tassy M, Marx M, Chow DS, Weinberg BD, Chang PD, Chaibi Y. Deep Learning-Based Algorithm for Automatic Detection of Pulmonary Embolism in Chest CT Angiograms. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13071324. [DOI] [PMC free article] [PubMed] [Google Scholar]