Abstract
This review focuses on the development and applications of organic polymer monoliths, with special attention to the literature published in 2021. The latest protocols in the preparation of polymer monoliths are discussed. In particular, tailored surface modification using nanomaterials, the development of chiral stationary phases and development of stationary phases for capillary electrochromatography are reviewed. Furthermore, the optimization of pore forming solvents composition is also discussed. Finally, the use of monolithic stationary phases in sample treatment using solid‐phase extraction and enrichment methods, molecularly imprinted polymers and enzymatic reactors is mentioned.
Keywords: column technology, liquid chromatography, monolith, sample treatment, surface modification
Abbreviations
- CEC
capillary electrochromatography
- CSP
chiral stationary phase
- GMA‐co‐EDMA
poly(glycidyl methacrylate‐co‐ethylene glycol dimethacrylate)
- MOFs
metal‐organic frameworks
- MIPs
Molecularly imprinted polymers
- MNPs
magnetite nanoparticles
- OT‐CEC
open tubular column applicable in CEC
- PEGDA
polyethylene glycol diacrylate
- SNW‐1
Schiff base network‐1
- SPME
solid‐phase microextraction
- VPBA
4‐vinylphenylboronic acid
1. INTRODUCTION
Pioneering work in the field of continuous stationary phases was carried out by Hjerten, 1 who prepared gel by polymerization of N,N´‐methylenebisacrylamide and acrylic acid in the presence of ammonium sulphate. In the next step, synthesized polymers have been compressed inside the column to form continuous separation media that has been applied in the separation of proteins. Independently, Tennikova and Belenkii collaborated with Švec 2 , 3 on the preparation of monolithic stationary phases in the format of flat polymer discs based on glycidyl methacrylate and ethylene dimethacrylate. In the early 1990s, Švec and Fréchet expanded this technology and introduced the first polymer monolithic chromatographic columns. 4 Due to their simple preparation, high permeability and wide pH stability, 5 , 6 organic polymer monoliths serve as an excellent alternative to the conventional particle columns 7 , 8 and, after 30 years of their development, have emerged as an important part of the family of stationary phases. 9
The preparation of polymer monolith is easy and straightforward. On the other hand, due to the large amount of experimental variables controlling the properties of the polymer (reaction time, temperature and composition of the polymerization mixture), the preparation of monolithic stationary phases yielding very good separation performance, requires a precisely tuned pore structure and chemistry. 10 , 11 The stainless steel column, fused‐silica capillary, or microfluidic channel is filled with a polymerization mixture containing functional and crosslinking monomers dissolved in porogenic solvents in the presence of a suitable polymerization reaction initiator. 3 The reaction is then initiated by elevated temperature or UV irradiation. Finally, the column is flushed to remove the unreacted components of the polymerization mixture. 12 The properties of monolithic stationary phases are easily controlled by the composition of the polymerization mixture, 3 polymerization temperature and reaction time 4 , 13 and post‐polymerization surface modification. 14
This review focuses on current trends and applications of polymer‐based monolithic stationary phases, summarizes the literature published in the year of 2021, and follows up to review article from the previous year. 15
2. CONTROLLING SURFACE CHEMISTRY AND MORPHOLOGY OF POLYMER MONOLITHS
The monolithic stationary phases may serve their purpose only if the surface chemistry corresponds to the desired application. For example, for reversed‐phase liquid chromatography, hydrophobic groups are required, ionisable groups must be present for the separation in ion‐exchange chromatography, and/or the presence of chiral selector in a stationary phase is necessary for chiral separations. 14 By carefully selecting the individual polymerization mixture components, the hydrodynamic and separation properties, such as porosity, efficiency, selectivity or permeability, can be controlled. 16
One possibility of preparing a monolithic stationary phase with the desired chemistry is direct copolymerization of crosslinking monomer with a selected functional monomer. Several functional monomers are available commercially, which significantly increases the diversity of possible surface chemistries. 17 Most of the columns are prepared using either methacrylate functional and crosslinking monomers 18 , 19 , 20 or styrene‐based monomers together with divinylbenzene. 21 , 22 , 23 Yet another option for how to prepare a monolithic stationary phase with the required surface chemistry is a post‐polymerization chemical modification based on the reaction of the reactive monolithic surface with derivative agents. For these modifications, reactive monomers, such as glycidyl methacrylate or vinylbenzyl chloride, are utilized the most. 24 A preferable way to achieve the required control of the surface chemistry of the monolith is grafting method. In this technique, functional monomer chains are attached to the surface of the generic monolith by radical polymerization initiated by elevated temperature or UV radiation. 17 The main advantage of grafting reaction is spatial control over the modification process and large variability in selecting functional monomers that might be used to alter the surface chemistry of a generic monolith.
2.1. Tailored surface modification
Polymer monolithic stationary phases are well known for separating large molecules. 2 On the other hand, preparation of polymer‐based monolithic stationary phases providing higher separation power for small molecules is still rather challenging. Several protocols were introduced to improve column efficiency 25 including early termination of polymerization reaction 26 , 27 , 28 or post‐polymerization surface modification with hyper‐cross‐linking reaction. 29 Recently, the selectivity and performance of organic polymer monoliths have been improved by the incorporation of different materials, such as metal‐organic frameworks (MOFs), 30 covalent organic frameworks, 31 or other types of nanostructured materials. 32 , 33 , 34 , 35 , 36 Polymer monoliths prepared by grafting modification reaction with MOFs and graphene oxide 37 were applied for CO2 capture and adsorption separation.
MOFs are porous materials consisting of the coordination of a metal ions or their clusters with organic ligand and exhibit unique properties including uniform pore sizes, large surface area and tunable surface chemistry. In combination with polymer monoliths, they allow the development of a novel type of composite monolithic stationary phases with improved chromatographic properties. Many immobilization approaches of MOFs onto organic polymer monoliths have been reported. 38 The easiest method how to integrate MOFs into the monolith structure is a direct addition of MOFs crystals in polymerization mixture, 39 where, however, blockage of polymer pores might result in retention loss and subsequent deterioration of analyte mass transfer. MOFs based on terephthalic acid and Zr(IV) (UiO‐66), 40 or Cr(III) (MIL‐101(Cr)), 41 were integrated into polymer monoliths structure and applied as stationary phases in SMPE for determination of penicillin analogues in water with extraction time being only 34 min. 42 Lately, a MIL‐53(Al)‐methacrylate composite monolithic capillary column for the reverse phase separation of alkylbenzenes and phenolic compounds has been developed. 43 Although the separation efficiency of these columns is still rather low, the composite materials provided improved chromatographic resolution of small aromatics when compared to bare monolith without any MOFs addition.
In chiral recognition of enantiomers, a complex of the analyte and the chiral stationary phase (CSP) is formed by means of various interactions, for example, hydrogen bond, dipole–dipole interaction, the steric effects, or the van der Waals forces. To describe chiral recognition of two enantiomers, “three‐point interaction model” has been introduced. 44 The incorporation of the NH2 group into the UiO‐66 MOF structure enhances the interactions with the chiral molecules and, thus, provides a binding site for the immobilization of the chiral selectors. 45 Direct thermal polymerization of poly(glycidyl methacrylate‐co‐ethylene glycol dimethacrylate) (GMA‐co‐EDMA) mixture with UiO‐66‐NH2 provided composite monolith that has been subsequently modified by the enzyme cellulase to allow chiral recognition. Poly(GMA‐co‐EDMA) monolith with cellulase, but without the presence of UiO‐66‐NH2 was prepared for a comparison. While monolith having in the structure both UiO‐66‐NH2 and the cellulose enzyme separated the racemic mixture of basic drugs metopropol, atenolol, esmolol, bisopropol and propranolol with resolutions of 1.67, 1.50, 1.52, 0.36, 0.44 in less than 15 min, the monolith prepared without MOF showed less cellulose immobilized onto the poly(GMA‐co‐EDMA) monolith and consequently provided poor chiral resolution.
Another way how to improve separation efficiency for small molecules is the development of polymer monolithic materials in combination with nanoparticles. 46 Several nanostructures have been used together with polymer monoliths during the last years including carbon nanotubes, 32 , 47 iron oxide nanoparticles, 48 and gold nanoparticles. 49 , 50 As is the case in the modification of polymer surface by MOFs, the incorporation of nanomaterials into polymer monoliths improves their separation efficiency. However, due to the low column efficiency of parent monolith, this improvement is only incremental and generally happens with higher concentrations of incorporated nanomaterials. 47 , 48
Torres‐Cartas et al. 46 prepared a monolithic micro‐column modified with magnetic nanoparticles and used the column to separate small phosphorylated compounds. The epoxy group of the generic GMA monolith was modified with magnetic nanoparticles functionalized with the amino group. This approach provided a reproducible layer of nanoparticles on a polymer monolith, as well as robust and permeable columns with sufficient efficiency for the separation of small molecules. Alternatively, a polymethacrylate‐based monolith with incorporated fumed silica nanoparticles that were functionalized with the covalent bond of carbamide moieties has been introduced. 51 A wide range of analytes, including neutral and polar low molecular weight solutes, nucleobases, nucleosides, organic acids, food additives, vitamins and biological amines, were well separated under HILIC conditions on the prepared carbamide‐fumed silica nanoparticles‐poly(glyceryl monomethacrylate‐co‐ethylene glycol dimethacrylate) monolith.
Chiral separations are a topic of concern in many areas such as pharmaceutical, agrochemical, environmental and food analysis. 52 , 53 , 54 Hence, the development of CSPs is very important for the successful resolution of enantiomers. In the last two decades, monolithic CSPs have received increased attention due to their natural advantages over traditional particle‐based materials such as high permeability and easy preparation within micro‐ or nanoformats. 12 , 55 , 56 The monolithic CSPs can be mainly classified based on the type of chiral selectors as cyclodextrin‐functionalized, polysaccharide‐functionalized, protein‐functionalized, antibiotic‐functionalized, chiral ligand‐exchange and chiral ion‐exchange. 57
Native or derivatised β‐cyclodextrine bonded to appropriate supports have been extensively used as chiral HPLC stationary phases 58 , 59 , 60 for the enantiomeric separation of optical isomers. 61 , 62 Recently, Zhao et al. 63 prepared a chiral monolithic stationary phase by direct polymerization of functional monomers allyl‐β‐cyclodextrin and methyl methacrylate together with triallyl isocyanurate and ethylene glycol dimethacrylate as crosslinking monomers. The monolithic stationary phase was prepared in a stainless‐steel column with a diameter of 4.6 mm and a length of 50 mm. The column showed good permeability and satisfactory efficiency (35,560 plates/m for naphthalene). The monolithic stationary phase was used to separate ibuprofen and ephedrine enantiomers with a resolution of 1.89 and 0.98.
Macrocyclic antibiotic daptomycin was used as a chiral selector to prepare a polymer monolithic stationary phase based on poly(GMA‐co‐EDMA) polymer. 64 Two approaches were tested to prepare a chiral monolithic stationary phase. In the first approach, generic poly(GMA‐co‐EDMA) monolith was flushed with 10 mg/mL of daptomycin for 24 and 48 h. The amino group in the structure of daptomycin reacted with a reactive epoxy group of GMA. In the second approach, daptomycin was added directly to the polymerization mixture. Daptomycin‐based monolithic stationary phases were tested for the enantioselective nano‐HPLC resolution of 50 racemic drugs of different pharmacological groups. While the monolith with daptomycin immobilized for 24‐h showed no significant separation of tested racemic mixtures, the monolith with 48‐h immobilization provided separation of 14 racemates with a resolution greater than 1. Finally, the monolith with encapsulated daptomycin provided enantioseparation of most tested compounds.
Trypsin is a proteolytic enzyme 65 that is generally immobilized on the surface of polymer monoliths to form a microfluidic enzymatic reactor. However, due to many chiral centres, trypsin can also act as a chiral selector. 66 Amalia et al. 67 prepared a poly(GMA‐co‐trimethylolpropane trimethacrylate) monolithic stationary phase with immobilized trypsin and used it to separate R/S citronellal enantiomers. Although baseline resolution was not achieved, it was possible to identify split peaks with different retention times. Hence, after a proper optimization, trypsin‐immobilized poly(GMA‐co‐trimethylolpropane trimethacrylate) monoliths show the potential to be applied in chiral separation of citronellal enantiomers.
Besides the pressure‐driven applications in liquid chromatography, polymer monoliths have also been applied in capillary electrochromatography (CEC) where the mobile phase is driven through the chromatographic bed by an electric field. 68 Neequaye et al. 69 prepared poly(carboxy ethyl acrylate‐co‐ethylene glycol dimethacrylate) monolithic stationary phase and n‐octadecyl (C18) ligands were subsequently bonded onto the surface by post‐polymerization functionalization. The resulting monolithic column exhibited a very low electro‐osmotic flow which required the addition of a small amount of 2‐acrylamido‐2‐methylpropane sulfonic acid in the polymerization mixture. The final nonpolar C18 monolithic column separated nonpolar and slightly polar compounds (e.g., alkylbenzenes, polyaromatic hydrocarbons and phenols) by reversed‐phase CEC. The highest efficiency was achieved for alkylbenzenes (57,800 plates/m), while the lowest column efficiency (15,590 plates/m) was observed for alkyl phenol ketones.
A novel open tubular column applicable in CEC (OT‐CEC) with a stable two‐layer copolymer as the stationary phase was prepared for peptides separation and provided a very high separation efficiency. 70 At first, a very thin monolithic monolayer of vinylbenzyl chloride and divinylbenzene has been grafted onto the inner capillary wall. Then, the second copolymer layer was formed on this thin monolithic monolayer by reversible addition‐fragmentation transfer polymerization of N‐phenylacrylamide and styrene. The resulting OT‐CEC columns, with an effective length of 1.1 m and a thickness of the polymer layer of about 2 μm provided 2.4 million theoretical plates per column for the mixture of six synthetic peptides. The high separation power of prepared capillary can be attributed to the formed double layer as electrophoretic separations performed on bare capillary and monolayer‐modified capillary provided either unsatisfactory resolution (bare capillary) or low separation efficiency (monolayer‐modified capillary) as shows in Figure 1.
FIGURE 1.

The chromatograms obtained with the OT‐CEC column: the acetone (A), bare capillary (B), monolayer deposited column (C) and two‐layer deposited column (D); mobile phase: 60:40 v/v ACN/20 mM ammonium formate at pH 6.0. Reprinted with permission from Ref. [ 70 ]
A monolithic capillary column modified with benzoic acid 71 was developed to separate polar compounds by capillary electrochromatography with a high content of water in the mobile phase. 4‐Vinylbenzoic acid was used as a functional monomer, where the benzene ring provides a strong hydrophobic interaction for retention, and carboxyl groups can be used as an electro‐osmotic flow generator. The column was prepared by direct polymerization of 4‐vinylbenzoic acid together with acryloyloxy‐3‐(methacryloyloxy)‐2‐propanol as a cross‐linking monomer. After a thorough optimization of the composition of the polymerization mixture, the column was characterized using scanning electron microscopy. The monolithic column showed good stability and repeatability and was used for the separation of analytes differing in polarity including short peptides and nucleoside bases. The highest column efficiency was determined for adenine (2,15,000 plates/m).
2.2. Porous properties of polymer monoliths
Porogenic solvents are a crucial part of the polymerization mixture, defining the porous properties of monolithic stationary phases such as monolith morphology, surface area, pore volume and permeability. Porogenic solvents control the porous properties of the monolith by solvation of polymer chains during the initial phases of the polymerization reaction and by the phase separation of the polymers formed within the polymerization mixture. 3 , 72 Solvents like methanol, 73 , 74 1‐propanol, 75 1,4‐butanediol, 76 , 77 hexane, 78 , 79 cyclohexanol, 80 , 81 decanol, 82 , 83 dodecanol, 84 , 85 and toluene, 81 , 86 have been extensively used in monolith preparations.
Several works have introduced new materials that could be used as porogenic solvents including solid porogens, 80 gaseous porogens, 87 , 88 , 89 or supercritical fluids. 87 Despite efforts to introduce new materials, liquid porogens are still dominantly used. In recent years, several new types of liquid porogenic solvents have been tested such as polymers, 90 ionic fluids, 84 and non‐ionic surfactants. 91 , 92
The main factor affecting porous properties of polymer monoliths, including average pore size, porosity and specific surface area, is the rate of phase separation in the polymerization process. The phase separation (process of macro‐porous structure formation) is controlled by (i) compatibility of the porogens and emerging polymer, (ii) volume of porogenic solvents, (iii) rate of the polymerization and (iv) the amount of crosslinking monomer. Korzhikova‐Vlakh and Tennikova 93 compared theoretical predictions of average pore size for different systems with an experimental data. The average pore size has been predicted by Hildebrand's and Hansen's solubility theories. In Hildebrand's theory, applicable in the description of nonpolar solvents behaviour, the solubility parameter is the square root of the cohesive energy density of the compound. In Hansen's theory, the Hildebrand's total solubility parameter is further subdivided into three terms: dispersion force, hydrogen bonding and polar parameter. Several polymer monoliths combining various monomer–porogenic solvents systems have been prepared by both thermally‐ and UV‐initiated polymerizations and determined average pore sizes were compared with values predicted by both theories. Authors concluded that while Hildebrand's theory cannot be used as universal tool in average pore size prediction, the Hansen's theory is helpful in the selection of porogenic solvents for the same system of monomers and the fixed monomers/porogens ratio. However, for the precise prediction of the average pore size, also additional factors, such as viscosity of the polymerization mixture, must be considered.
Mansour et al. 94 focused on the addition of various types of non‐ionic surfactants in polyethylene glycol diacrylate (PEGDA)‐based monoliths. They tested the hypothesis that the addition of surfactants can form a “universal” porogen solvents system. The authors tried eight different surfactants that differed in physical properties, chemical structures and molecular weights. The prepared monolithic columns were characterized by scanning electron microscopy (Figure 2). Only four of the used surfactants provided permeable monoliths that were further chromatographically tested with a mixture of alkylbenzenes in the acetonitrile/water mobile phase (20:80, v/v). The highest efficiency, 17,280 plates/m and methylene selectivity of 1.46 were observed when the porogenic mixture contained 30% Tween 40, 20% decan, 15% decanol and 15% dodecanol. A selected porogenic mixture was further used in the preparation of a series of monolithic columns with various methacrylate‐ and styrene‐based monomers. Columns prepared by copolymerization of PEGDA with lauryl methacrylate and butyl methacrylate and the above‐mentioned porogenic system showed the best efficiencies, 30,500 and 27,700 plates/m.
FIGURE 2.

Scanning electron microscope images of the monolith prepared from poly(ethylene glycol) diacrylate together with different non‐ionic surfactants: C1–polyoxyethylene (4) lauryl ether (Brij L4), C2–sorbitan monooleate (SPAN 80), C3–polyoxyethylene (5) nonylphenylether (IGEPAL CO‐520), C4–polyoxyethylene (9) 1‐pentyloctylether (Tergitol 15S9), C5–2,4,7,9‐tetramethyl‐5‐decyne‐4,7‐diol ethoxylate (TMDDE),C6–polyoxyethylenesorbitan monopalmitate (Tween 40), C6–octylphenol ethoxylate (Triton X‐405), C7–ethylenediamine tetrakis(propoxylate‐block‐ethoxylate) tetrol (Tetronic 701). Reprinted with permission from Ref. [ 94 ]
Based on these results, the same group explored an effect of chain length, degree of substitution and saturation of Tween surfactant (Tween 20, 40, 60, 80, 85) on the morphology of PEGDA columns and the chromatographic efficiency for small molecules. 95 After a comprehensive study combining scanning electron microscopy, surface area, elemental analysis and infrared spectrometry, they found that column with the addition of 30% w/w of Tween 60 showed the best performance with column efficiency of 23,960 plates/m. Moreover, when PEGDA concentration increased while keeping Tween 60 concentration constant, the column efficiency also increased at the expense of the lower column permeability. Finally, the optimized column prepared with 30% PEGDA, 30% Tween 60, 16% decanol and 12% dodecanol in the polymerization mixture provided the highest column efficiency (60,000 plates/m).
These results demonstrate that column efficiency of polymer monoliths can be easily tuned and controlled by the composition of the polymerization mixture. However, obtained efficiencies are far below efficiency of currently available columns packed with superficially porous and sub‐2 μm particles. 9 On the other hand, almost unlimited variability in the preparation of polymer monoliths provides no limits in further development and makes them very versatile separation materials.
3. MONOLITHIC MATERIALS FOR SAMPLE PREPARATION
3.1. Enrichment and solid‐phase extraction
Besides the main application of polymer monoliths in liquid chromatography separations, they can also be applied in the field of sample preparation, 96 where solid‐phase microextraction (SPME) is one of the most preferred techniques with high efficiency, speed and low solvent consumption. 97
Hu et al. 98 were the first who coupled online in‐tube SPME with mass spectrometry, where monolithic SPME capillary served as the electrospray emitter. The scheme of the developed device is shown in Figure 3. The ionic liquid‐based monolithic SPME capillary was prepared by copolymerization of 1‐allyl‐methylimidazolium chloride and ethylene glycol dimethacrylate. Highly porous organic polymer provided excellent extraction efficiency toward hydrophobic and anionic compounds and the formation of smaller electrospray droplets also increased the ionization efficiency and stability. An online in‐tube SPME mass spectrometry system was applied to quantify two non‐steroid anti‐inflammatory drugs in plasma and urine with significant pre‐concentration ability and improved efficiency of electrospray ionization. Recoveries ranges for ketoprofen and flurbiprofen determined by spiking of the plasma and urine samples were 86.0−118.5% and 92.4−109.5%, respectively. The values of recoveries significantly exceeding 100% can be–at least to some extent–explained by possible nonspecific sorption or by inhomogeneity in electrospray formation, as they increased at higher concentration of added analyte during spiking analysis.
FIGURE 3.

Diagram of the online in‐tube SPME‐MS system. The sample solution was injected by a sampling pump, and the washing solution of water was infused by the sampling pump using different syringes. The desorption solution was pumped by the other valve. Reprinted with permission from Ref. [ 98 ]
As is the case in liquid chromatography‐based separations, modification of polymer monoliths with nanoparticles is also important in the development of materials for sample preparation methods. Wang et al. 99 described the preparation of a new monolithic column with the incorporation of Schiff base network‐1 (SNW‐1) nanoparticles for the extraction of antiepileptic drugs. The column was prepared by direct thermal copolymerization of the SNW‐1 nanoparticles together with GMA and EDMA in the presence of porogenic solvents and initiator. Subsequently, the monolithic column was coupled to the HPLC system for an online extraction and detection of antiepileptic drugs. The online method provided good linearity, low limits of detection (0.2 ng/mL) and good repeatability for the determination of three antiepileptic drugs in an aqueous solution. The developed method was also successfully applied to determine antiepileptic drugs in human plasma. Recoveries determined by spiking of the plasma sample with the analyte were in the range 88.6–106.1%. Zhang et al. 100 developed a monolithic capillary column for highly selective recognition of patulin mycotoxin. They prepared a poly(GMA‐co‐PEGDA) monolith modified by gold nanoparticles. Subsequently, the column was filled with patulin aptamer, and the column was incubated for 10 h at 37°C. The prepared column was used to determine patulin in food samples (e.g., apple, orange and pear juice) and provided sensitive and selective determination of patulin with method recoveries of 85.4–106%, determined by spiking of the sample with patulin.
Protein glycosylation and phosphorylation are some of the most important protein post‐translational modifications. Several approaches have been developed for the enrichment of glycoproteins 101 or phosphoproteins. 102 Recently, preparation of a microbore monolithic column modified with magnetite nanoparticles (MNPs) was successfully applied to enrich phosphopeptides from a tryptic digest of β‐casein. 46 The column was prepared by the covalent attachment of amino‐modified MNPs to the polymer surface of generic GMA monolith. As shown in Figure 4, the direct analysis of β‐casein digest provided detection of only one phosphopeptide while the signals of non‐phosphorylated peptides substantially decreased after enrichment and resulted in the detection of five phosphopeptides in the MS spectra.
FIGURE 4.
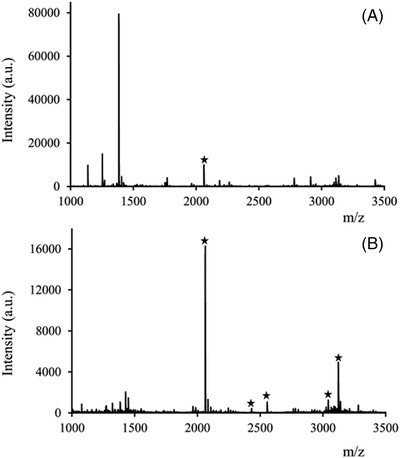
MALDI‐TOF mass spectra of tryptic digest of β‐casein without (A) and with phosphopeptide enrichment using the hybrid monolithic column (B). Reprinted with permission from Ref. [ 46 ]
Ali et al. 103 used attachment of 5‐boronoisophthalic acid to the GMA‐based polymer surface as a novel affinity ligand allowing the selective capture and release of glycoproteins. Boronic acid functionality covalently bonds to cis‐diol containing biomolecules in dependence on pH. In an alkaline environment, boronic acid forms an ester ring with cis‐diol compounds, while in acidic pH, this bond dissociates. 104 The resulting affinity material was packed in a micropipette tip and applied to selectively enrich glycoproteins, especially lactoferrin, from the human milk.
Using the same reaction of boronic acid functionality, the synthesis of a bi‐functional polymer monolithic column allowing the specific capture of both glycoproteins and phosphopeptides was described by Huang et al. 105 The column was prepared by direct polymerization of 4‐vinylphenylboronic acid (VPBA) and vinylphosphonic acid with EDMA cross‐linker. Boronate affinity moieties (VPBA) provided specific recognition towards cis‐diol containing glycoproteins in alkaline conditions. To recognize molecules containing phosphate groups, the column was first flushed with ZrCl4 to form Zr4+‐phosphate coordination. When zirconium ion was immobilized on the monoliths, the biomolecules containing phosphate groups were selectively captured on immobilized metal affinity chromatography in acidic conditions.
3.2. Molecularly imprinted polymers
Molecularly imprinted polymers (MIPs) are highly selective materials applicable in chromatographic or electrophoretic separations and/or sample preparation. MIPs are synthetic polymers prepared by copolymerization of functional and crosslinking monomers in the presence of a template molecule. After polymerization, the template molecule is removed, causing the formation of selective recognition sites that can subsequently bind the targeted molecules. 106 The preparation procedures for MIPs in different formats and application fields were widely reviewed last year with a particular focus on chiral MIPs, 107 sensors for cancer biomarkers, 108 and determination of DNA, 109 analysis of protein biomarkers, 110 and their applications in food safety. 111
One of the efforts in current analytical chemistry is the miniaturization of analytical systems and integration of individual steps combining sample preparation, separation, and detection. MIPs can be advantageously integrated into miniaturized SPE devices, 112 , 113 , 114 , 115 where monolithic imprinted polymers can be synthesized directly into capillaries or chip microchannels. 112 Recently, the preparation of MIPs in miniaturized devices 112 has also been reviewed.
Bouvarel et al. 116 prepared miniaturized monolithic MIPs in a capillary with an internal diameter of 100 μm for selective capture of benzoylecgonine, the primary urinary metabolite of cocaine. The monolithic MIP was prepared by photopolymerizing methacrylic acid, trimethylolpropane trimethacrylate, isooctane and cocaine as a template molecule. After washing out the template, the capillary MIP was online coupled to UV detection. Optimization of experimental conditions (e.g., column length and extraction conditions) allowed successful extraction of benzoylecgonine from human urine with limits of detection and quantification of 56.4 and 188.0 ng/mL, respectively.
A MIP in a capillary with an internal diameter of 100 μm was also used for extraction and online detection of ochratoxin A in the beer sample. 117 The MIPs were prepared by the radical polymerization reaction of acrylamide‐2‐methylpropanesulfonic acid, methacrylate substituted polyhedral oligomeric silsesquioxanes, ethylene glycol dimethacrylate and ochratoxin A as a template molecule. The recovery rate of MIP for ochratoxin A was determined to be 84.8%, while non‐imprinted polymer provided only 4.5%.
3.3. Enzymatic reactors
Due to the easy preparation, simple surface modification and favourable highly porous internal structure, polymer‐based monolithic stationary phases successfully serve as a support for enzyme immobilization. 118 Recently, several reviews dealing with the preparation of enzymatic reactors based on polymer monoliths have been published. 119 , 120 , 121
Trypsin‐immobilized poly (GMA‐co‐EDMA) monolithic micro reactor for online digestion of β‐casein was prepared by Amalia et al. 67 The effect of the digestion flow rate and the temperature of digestion were investigated to obtain the optimum interaction between the protein and the immobilized trypsin. The number of appeared peaks, as well as the peak intensities at the digestion flow rate of 1 μL/min (residence time 80 min) was higher than that in a flow rate of 5 μL/min (residence time 16 min) and 10 μL/min (residence time 8 min). It was also found that the digestion temperature of 45–50°C provided better conversion of the proteins into peptides than the temperature of 37°C, indicated by higher peak intensities as well as a higher number of identified peaks. A higher digestion temperature is probably advantageous due to the formed temperature gradient in between the interior of the reactor and the outside environment.
A microfluidic platform (Figure 5) reported by Wei et al. 122 integrated online protein fractionation, denaturation, digestion and peptide enrichment in a single microfluidic device. The protein sample was first reduced by dithiothreitol and then alkylated by the iodoacetamide in a microfluidic platform, and the denatured sample was then transferred to an enzymatic reactor with trypsin immobilized into the skeleton of the polytrimethylolpropane trimethacrylate monolith to ensure protein digestion of MCF‐7 cells. The immobilized trypsin reactor based on polytrimethylolpropane trimethacrylate monolith proved to be very stable with maintaining more than 86% of enzyme activity when stored at 4°C for 2 months.
FIGURE 5.

The overview of the integrated microfluidic system. (A) Proteins fractionation and online treatment, (B) elution of proteins, (C) online digestion of eluted proteins. AP–polyallyl phenoxyacetate, DTT–dithiothreitol, IAA–iodoacetamide. Reprinted with permission from Ref. [ 122 ]
4. SUMMARY AND OUTLOOK
This review highlights current trends and the newest approaches in preparing and utilizing organic polymer monolithic materials for the year 2021. Monoliths are often used as prepared by direct copolymerization; however, the tailored modification of their surface usually provides materials with new properties. In recent years, the selectivity and efficiency of polymer monoliths have been improved by the introduction of MOFs or nanoparticles with large surface area, high porosity and easy functionalization, leading to composite materials applicable as stationary phases. Thanks to their simple preparation and surface modification, the monolithic stationary phases were also prepared as CSPs, even at micro‐ or nanoscale.
In addition to the selection of monomers in the polymerization mixture, the properties of monoliths can be controlled by the composition of the porogenic solvents. Although proven systems of the liquid porogenic solvent system still prevail, new combinations that change the porous properties of monoliths, such as morphology, surface area and permeability, have also appeared recently.
The application of monolithic stationary phases is dominant in liquid chromatography‐based separations. However, they can also be successfully applied in the field of sample preparation as demonstrated in this review. For example, the in‐tube SPME capillary was used directly as an electrospray emitter for connection with mass spectrometry. Monolithic materials also allow the immobilization of enzymes and, thus, facilitate the preparation of enzymatic reactors for proteomic analysis. The ultimate advantage of monoliths is their easy miniaturization, providing the preparation of integrated systems that allow multiple‐step sample treatment in one device, which dramatically reduces the consumption of chemicals and the time required for sample preparation.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
AUTHORS’ CONTRIBUTION
Martina Nechvátalová Conceptualization, Writing Original Draft, Writing‐Review & Editing.
Jiří Urban–Writing‐Review & Editing, Funding acquisition.
ACKNOWLEDGMENT
The financial support of Czech Science Foundation project 20‐21903S is gratefully acknowledged.
Nechvátalová M, Urban J. Current trends in the development of polymer‐based monolithic stationary phases. Anal Sci Adv. 2022;3:154–164. 10.1002/ansa.202100065
DATA AVAILABILITY STATEMENT
Data sharing not applicable–no new data generated.
REFERENCES
- 1. Hjerten S, Liao J. High‐performance liquid‐chromatography of proteins on compressed, non‐porous agarose beads .1. Hydrophobic‐interaction chromatography. Journal of Chromatography. 1988;457:165‐174. [DOI] [PubMed] [Google Scholar]
- 2. Tennikova T, Bleha M, Svec F, Almazova T, Belenkii B. High‐performance membrane chromatography of proteins, a novel method of protein separation. Journal of Chromatography. 1991;555(1‐2):97‐107. [Google Scholar]
- 3. Svec F. Porous polymer monoliths: amazingly wide variety of techniques enabling their preparation. J Chromatogr A. 2010;1217(6):902‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frantisek Svec, Frechet JMJ. Continuous rods of macroporous polymer as high‐performance liquid chromatography separation media. Anal Chem. 1992;64(7):820‐822. [DOI] [PubMed] [Google Scholar]
- 5. Svec F, Lv Y. Advances and recent trends in the field of monolithic columns for chromatography. Anal Chem. 2015;87(1):250‐273. [DOI] [PubMed] [Google Scholar]
- 6. Ibrahim AE, Hashem H, Saleh H, Elhenawee M. Performance comparison between monolithic, core‐shell, and totally porous particulate columns for application in greener and faster chromatography. J AOAC Int. 2018;101(6):1985‐1992. [DOI] [PubMed] [Google Scholar]
- 7. Peters EC, Petro M, Svec F, Frechet JMJ. Molded rigid polymer monoliths as separation media for capillary electrochromatography. 1. Fine control of porous properties and surface chemistry. Anal Chem. 1998;70(11):2288‐2295. [DOI] [PubMed] [Google Scholar]
- 8. Peters EC, Petro M, Svec F, Fréchet JMJ. Molded rigid polymer monoliths as separation media for capillary electrochromatography. 2. Effect of chromatographic conditions on the separation. Anal Chem. 1998;70(11):2296‐2302. [DOI] [PubMed] [Google Scholar]
- 9. Urban J. Are we approaching a post‐monolithic era? J Sep Sci. 2020;43(9‐10):1628‐1633. [DOI] [PubMed] [Google Scholar]
- 10. Komendova M, Ribeiro LF, Urban J. Controlling selectivity of polymer‐based monolithic stationary phases. J Sep Sci. 2019;42(5):952‐961. [DOI] [PubMed] [Google Scholar]
- 11. Dores‐Sousa JL, Terryn H, Eeltink S. Morphology optimization and assessment of the performance limits of high‐porosity nanostructured polymer monolithic capillary columns for proteomics analysis. Analytica Chimica Acta. 2020;1124:176‐183. [DOI] [PubMed] [Google Scholar]
- 12. Zou HF, Huang XD, Ye ML, Luo QZ. Monolithic stationary phases for liquid chromatography and capillary electrochromatography. J Chromatogr A. 2002;954(1‐2):5‐32. [DOI] [PubMed] [Google Scholar]
- 13. Tennikova T, Belenkii B, Svec F. High‐performance membrane chromatography—a novel method of protein separation. J Liq Chromatogr. 1990;13(1):63‐70. [Google Scholar]
- 14. Svec F. Preparation and HPLC applications of rigid macroporous organic polymer monoliths. J Sep Science. 2004;27(10‐11):747‐766. [DOI] [PubMed] [Google Scholar]
- 15. Eeltink S, Meston D, Svec F. Recent developments and applications of polymer monolithic stationary phases. Analytical Science Advances. 2021;2(3‐4):250‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Svec F, Peters EC, Sýkora D, Yu C, Fréchet JMJ. Monolithic stationary phases for capillary electrochromatography based on synthetic polymers: designs and applications. J High Resolut Chromatograph. 2000;23(1):3‐18. [Google Scholar]
- 17. Viklund C, Svec F, Frechet JMJ, Irgum K. Fast ion‐exchange HPLC of proteins using porous poly(glycidyl methacrylate‐co‐ethylene dimethacrylate) monoliths grafted with poly(2‐acrylamido‐2‐methyl‐1‐propanesulfonic acid). Biotechnol Prog. 1997;13(5):597‐600. [DOI] [PubMed] [Google Scholar]
- 18. Coufal P, Čihák M, Suchánková J, Tesařová E, Bosáková Z, Štulík K. Methacrylate monolithic columns of 320 μm I.D. for capillary liquid chromatography. Journal of Chromatography A. 2002;946(1‐2):99‐106. [DOI] [PubMed] [Google Scholar]
- 19. Moravcova D, Jandera P, Urban J, Planeta J. Characterization of polymer monolithic stationary phases for capillary HPLC. J Sep Sci. 2003;26(11):1005‐1016. [Google Scholar]
- 20. Moravcová D, Jandera P, Urban J, Planeta J. Comparison of monolithic silica and polymethacrylate capillary columns for LC. Journal of Separation Science. 2004;27(10‐11):789‐800. [DOI] [PubMed] [Google Scholar]
- 21. Gusev I, Huang X, Horvath C. Capillary columns with in situ formed porous monolithic packing for micro high‐performance liquid chromatography and capillary electrochromatography. J Chromatogr A. 1999;855(1):273‐290. [DOI] [PubMed] [Google Scholar]
- 22. Kucerova Z, Szumski M, Buszewski B, Jandera P. Alkylated poly(styrene‐divinylbenzene) monolithic columns for mu‐HPLC and CEC separation of phenolic acids. J Sep Sci. 2007;30(17):3018‐3026. [DOI] [PubMed] [Google Scholar]
- 23. Svobodova A, Krizek T, Sirc J, et al. Monolithic columns based on a poly(styrene‐divinylbenzene‐methacrylic acid) copolymer for capillary liquid chromatography of small organic molecules. J Chromatogr A. 2011;1218(11):1544‐1547. [DOI] [PubMed] [Google Scholar]
- 24. Xie S, Allington RW, Fréchet JMJ, Svec F, Freitag R. Modern advances in chromatography. Advances in Biochemical Engineering/Biotechnology. Berlin: Springer; 2002:87‐125. [Google Scholar]
- 25. Urban J. Current trends in the development of porous polymer monoliths for the separation of small molecules. J Sep Sci. 2016;39(1):51‐68. [DOI] [PubMed] [Google Scholar]
- 26. Nischang I, Brueggemann O. On the separation of small molecules by means of nano‐liquid chromatography with methacrylate‐based macroporous polymer monoliths. J Chromatogr A. 2010;1217(33):5389‐5397. [DOI] [PubMed] [Google Scholar]
- 27. Nischang I, Teasdale I, Brüggemann O. Towards porous polymer monoliths for the efficient, retention‐independent performance in the isocratic separation of small molecules by means of nano‐liquid chromatography. Journal of Chromatography A. 2010;1217(48):7514‐7522. [DOI] [PubMed] [Google Scholar]
- 28. Nischang I. Porous polymer monoliths: morphology, porous properties, polymer nanoscale gel structure and their impact on chromatographic performance. Journal of Chromatography A. 2013;1287:39‐58. [DOI] [PubMed] [Google Scholar]
- 29. Urban J, Svec F, Fréchet JMJ. Efficient separation of small molecules using a large surface area hypercrosslinked monolithic polymer capillary column. Anal Chem. 2010;82(5):1621‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furukawa H, Cordova KE, O'Keeffe M, Yaghi OM. The chemistry and applications of metal‐organic frameworks. Science. 2013;341(6149):974. [DOI] [PubMed] [Google Scholar]
- 31. Cote AP, Benin AI, Ockwig NW, O'Keeffe M, Matzger AJ, Yaghi OM. Porous, crystalline, covalent organic frameworks. Science. 2005;310(5751):1166‐1170. [DOI] [PubMed] [Google Scholar]
- 32. Chambers SD, Svec F, Frechet JMJ. Incorporation of carbon nanotubes in porous polymer monolithic capillary columns to enhance the chromatographic separation of small molecules. J Chromatogr A. 2011;1218(18):2546‐2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peristyy AA, Fedyanina ON, Paull B, Nesterenko PN. Diamond based adsorbents and their application in chromatography. J Chromatogr A. 2014;1357:68‐86. [DOI] [PubMed] [Google Scholar]
- 34. Long DL, Tsunashima R, Polyoxometalates CroninL. Building blocks for functional nanoscale systems. Angew Chem‐Int Edit. 2010;49(10):1736‐1758. [DOI] [PubMed] [Google Scholar]
- 35. Sajid M, Basheer C. Layered double hydroxides: emerging sorbent materials for analytical extractions. Trac‐Trends Anal Chem. 2016;75:174‐182. [Google Scholar]
- 36. Wang T, Chen Y, Ma J, et al. Attapulgite nanoparticles‐modified monolithic column for hydrophilic in‐tube solid‐phase microextraction of cyromazine and melamine. Anal Chem. 2016;88(3):1535‐1541. [DOI] [PubMed] [Google Scholar]
- 37. Ning H, Yang Z, Yin Z, et al. A novel strategy to enhance the performance of CO2 adsorption separation: grafting hyper‐cross‐linked polyimide onto composites of UiO‐66‐NH2 and GO. ACS Appl Mater Interfaces. 2021;13(15):17781‐17790. [DOI] [PubMed] [Google Scholar]
- 38. Lv Y, Tan X, Svec F. Preparation and applications of monolithic structures containing metal‐organic frameworks. J Sep Sci. 2017;40(1):272‐287. [DOI] [PubMed] [Google Scholar]
- 39. Maya F, Paull B. Recent strategies to enhance the performance of polymer monoliths for analytical separations. J Sep Sci. 2019;42(8):1564‐1576. [DOI] [PubMed] [Google Scholar]
- 40. Fu YY, Yang CX, Yan XP. Incorporation of metal‐organic framework UiO‐66 into porous polymer monoliths to enhance the liquid chromatographic separation of small molecules. Chem Commun. 2013;49(64):7162‐7164. [DOI] [PubMed] [Google Scholar]
- 41. Huang HY, Lin CL, Wu CY, Cheng YJ, Lin CH. Metal organic framework‐organic polymer monolith stationary phases for capillary electrochromatography and nano‐liquid chromatography. Anal Chim Acta. 2013;779:96‐103. [DOI] [PubMed] [Google Scholar]
- 42. Lin CL, Lirio S, Chen YT, Lin CH, Huang HY. A novel hybrid metal‐organic framework‐polymeric monolith for solid‐phase microextraction. Chem Eur J. 2014;20(12):3317‐3321. [DOI] [PubMed] [Google Scholar]
- 43. Yusuf K, Badjah‐Hadj‐Ahmed AY, Aqel A, ALOthman ZA. Monolithic metal‐organic framework MIL‐53(Al)‐polymethacrylate composite column for the reversed‐phase capillary liquid chromatography separation of small aromatics. J Sep Sci. 2016;39(5):880‐888. [DOI] [PubMed] [Google Scholar]
- 44. Snyder LR, Kirkland JJ, Dolan JW. Introduction to Modern Liquid Chromatography, 3rd ed. New York, NY: Wiley. Accessed February 10, 2022. https://www.wiley.com/en‐ie/Introduction+to+Modern+Liquid+Chromatography%2C+3rd+Edition‐p‐9780470167540. [Google Scholar]
- 45. Ma M, Zhang J, Li P, et al. Immobilization of cellulase on monolith supported with Zr(IV)‐based metal‐organic framework as chiral stationary phase for enantioseparation of five basic drugs in capillary electrochromatography. Microchim Acta. 2021;188(6):186. [DOI] [PubMed] [Google Scholar]
- 46. Torres‐Cartas S, Meseguer‐Lloret S, Gomez‐Benito C, Catala‐Icardo M, Simo‐Alfonso EF, Manuel Herrero‐Martinez J. Preparation of monolithic polymer‐magnetite nanoparticle composites into poly(ethylene‐co‐tetrafluoroethylene) tubes for uses in micro‐bore HPLC separation and extraction of phosphorylated compounds. Talanta. 2021;224:121806. [DOI] [PubMed] [Google Scholar]
- 47. Fresco‐Cala B, Carrasco‐Correa EJ, Cárdenas S, Herrero‐Martínez JM. Carbon nanostructures incorporated on methacrylate monoliths for separation of small molecules by nano‐liquid chromatography. Microchemical Journal. 2018;139:222‐229. [Google Scholar]
- 48. Javier Carrasco‐Correa E, Ramis‐Ramos G. Hybrid methacrylate monolithic columns containing magnetic nanoparticles for capillary electrochromatography. J Chromatogr A. 2015;1385:77‐84. [DOI] [PubMed] [Google Scholar]
- 49. Lv Y, Alejandro FM, Frechet JMJ, Svec F. Preparation of porous polymer monoliths featuring enhanced surface coverage with gold nanoparticles. J Chromatogr A. 2012;1261:121‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terborg L, Masini JC, Lin M, Lipponen K, Riekolla ML, Svec F. Porous polymer monolithic columns with gold nanoparticles as an intermediate ligand for the separation of proteins in reverse phase‐ion exchange mixed mode. J Adv Res. 2015;6(3):441‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ganewatta N, El Rassi Z. Polymethacrylate‐based monolithic column with incorporated carbamide‐modified fumed silica nanoparticles for hydrophilic liquid interaction chromatography. J Liq Chromatogr Relat Technol. 2021;44(3‐4):255‐264. [Google Scholar]
- 52. Ward TJ, Ward KD. Chiral separations: a review of current topics and trends. Anal Chem. 2012;84(2):626‐635. [DOI] [PubMed] [Google Scholar]
- 53. Eljarrat E, Guerra P, Barcelo D. Enantiomeric determination of chiral persistent organic pollutants and their metabolites. Trac‐Trends Anal Chem. 2008;27(10):847‐861. [Google Scholar]
- 54. Rocco A, Aturki Z, Fanali S. Chiral separations in food analysis. TrAC Trends in Analytical Chemistry. 2013;52:206‐225. [Google Scholar]
- 55. Svec F. Recent developments in the field of monolithic stationary phases for capillary electrochromatography. J Sep Sci. 2005;28(8):729‐745. [DOI] [PubMed] [Google Scholar]
- 56. Tanaka N, Kobayashi H, Ishizuka N, et al. Monolithic silica columns for high‐efficiency chromatographic separations. J Chromatogr A. 2002;965(1‐2):35‐49. [DOI] [PubMed] [Google Scholar]
- 57. Guo J, Wang Q, Xu D, Crommen J, Jiang Z. Recent advances in preparation and applications of monolithic chiral stationary phases. TrAC Trends in Analytical Chemistry. 2020;123:115774. [Google Scholar]
- 58. Chen L, Li M, Ai Y, Dang X, Huang J, Chen H. One‐pot preparation of an acryloyled beta‐cyclodextrin‐silica hybrid monolithic column and its application for determination of carbendazim and carbaryl. Food Chem. 2018;269:181‐186. [DOI] [PubMed] [Google Scholar]
- 59. Shen J, Okamoto Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem Rev. 2016;116(3):1094‐1138. [DOI] [PubMed] [Google Scholar]
- 60. Deng M, Li M, Zhao Y, Jiang Z, Guo X. A novel one‐pot strategy to prepare beta‐cyclodextrin functionalized capillary monoliths for enantioseparation of basic drugs. Talanta. 2018;189:458‐466. [DOI] [PubMed] [Google Scholar]
- 61. Ahmed M, Ghanem A. Chiral β‐cyclodextrin functionalized polymer monolith for the direct enantioselective reversed phase nano liquid chromatographic separation of racemic pharmaceuticals. Journal of Chromatography A. 2014;1345:115‐127. [DOI] [PubMed] [Google Scholar]
- 62. Park JM, Park JH. Enantiomer separations of basic chiral compounds by capillary electrochromatography on a phosphated β‐cyclodextrin‐modified zirconia monolith. Journal of Chromatography A. 2014;1339:229‐233. [DOI] [PubMed] [Google Scholar]
- 63. Zhao Y, Si H, Zhao X, et al. Fabrication of an allyl‐β‐cyclodextrin based monolithic column with triallyl isocyanurate as co‐crosslinker and its application in separation of lipopeptide antibiotics by HPLC. Microchemical Journal. 2021;168:106462. [Google Scholar]
- 64. Fouad A, Marzouk AA, Shaykoon MSA, Ibrahim SM, El‐Adl SM, Ghanem A. Daptomycin: a novel macrocyclic antibiotic as a chiral selector in an organic polymer monolithic capillary for the enantioselective analysis of a set of pharmaceuticals. Molecules. 2021;26(12):3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Calleri E, Temporini C, Perani E, et al. Development of a bioreactor based on trypsin immobilized on monolithic support for the on‐line digestion and identification of proteins. Journal of Chromatography A. 2004;1045(1):99‐109. [DOI] [PubMed] [Google Scholar]
- 66. Thelohan S, Jadaud P, Wainer IW. Immobilized enzymes as chromatographic phases for HPLC: the chromatography of free and derivatized amino acids on immobilized trypsin. Chromatographia. 1989;28(11‐12):551‐555. [Google Scholar]
- 67. Amalia S, Angga SC, Iftitah ED, et al. Immobilization of trypsin onto porous methacrylate‐based monolith for flow‐through protein digestion and its potential application to chiral separation using liquid chromatography. Heliyon. 2021;7(8):e07707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rathore AS, Horváth C. Chapter 1‐Migration of charged sample components and electroosmotic flow in packed capillary columns. Journal of Chromatography Library. 2001;62:1‐38. [Google Scholar]
- 69. Neequaye T, El Rassi Z. Poly(carboxyethyl acrylate‐co‐ethylene glycol dimethacrylate) precursor monolith with bonded octadecyl ligands for use in reversed‐phase capillary electrochromatography. Electrophoresis. 2021;42:2656–2663. [DOI] [PubMed] [Google Scholar]
- 70. Sun G, Tang W, Lu Y, Row KH. Growth of two‐layer copolymer as the stationary phase with very high separation efficiency for separating peptides in capillary electrochromatography. Electrophoresis. 2021;42(20):2087‐2093. [DOI] [PubMed] [Google Scholar]
- 71. Hu C, Mao Z, Li Z, Li Q, Chen Z. Benzoic acid‐modified monolithic column for separation of hydrophilic compounds by capillary electrochromatography with high content of water in mobile phase. J Chromatogr A. 2021;1647:462166. [DOI] [PubMed] [Google Scholar]
- 72. Svec F, Frechet J. Kinetic control of pore formation in macroporous polymers—formation of molded porous materials with high‐flow characteristics for separations or catalysis. Chem Mat. 1995;7(4):707‐715. [Google Scholar]
- 73. Li Y, Tolley HD, Lee ML. Poly[hydroxyethyl acrylate‐co‐poly(ethylene glycol) diacrylate] monolithic column for efficient hydrophobic interaction chromatography of proteins. Anal Chem. 2009;81(22):9416‐9424. [DOI] [PubMed] [Google Scholar]
- 74. Liu Z, Peng Y, Wang T, et al. Preparation and application of novel zwitterionic monolithic column for hydrophilic interaction chromatography. Journal of Separation Science. 2013;36(2):262‐269. [DOI] [PubMed] [Google Scholar]
- 75. Wang J, Jiang X, Zhang H, Liu S, Bai L, Liu H. Preparation of a porous polymer monolithic column with an ionic liquid as a porogen and its applications for the separation of small molecules in high performance liquid chromatography. Anal Methods. 2015;7(18):7879‐7888. [Google Scholar]
- 76. Urban J, Jandera P, Langmaier P. Effects of functional monomers on retention behavior of small and large molecules in monolithic capillary columns at isocratic and gradient conditions. J Sep Sci. 2011;34(16‐17):2054‐2062. [DOI] [PubMed] [Google Scholar]
- 77. Gu C, He J, Jia J, Fang N, Simmons R, Shamsi SA. Surfactant‐bound monolithic columns for separation of proteins in capillary high performance liquid chromatography. J Chromatogr A. 2010;1217(4):530‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Gu B, Tolley HD, Lee ML. Preparation of polymeric monoliths by copolymerization of acrylate monomers with amine functionalities for anion‐exchange capillary liquid chromatography of proteins. J Chromatogr A. 2009;1216(29):5525‐5532. [DOI] [PubMed] [Google Scholar]
- 79. Santora BP, Gagné MR, Moloy KG, Radu NS. Porogen and cross‐linking effects on the surface area, pore volume distribution, and morphology of macroporous polymers obtained by bulk polymerization. Macromolecules. 2001;34(3):658‐661. [Google Scholar]
- 80. Du KF, Yang D, Sun Y. Fabrication of high‐permeability and high‐capacity monolith for protein chromatography. J Chromatogr A. 2007;1163(1‐2):212‐218. [DOI] [PubMed] [Google Scholar]
- 81. Zhong H, El Rassi Z. Neutral polar methacrylate‐based monoliths for normal phase nano‐LC and CEC of polar species including N‐glycans. J Sep Sci. 2009;32(1):10‐20. [DOI] [PubMed] [Google Scholar]
- 82. Koeck R, Fischnaller M, Bakry R, Tessadri R, Bonn GK. Preparation and evaluation of monolithic poly(N‐vinylcarbazole‐co‐1,4‐divinylbenzene) capillary columns for the separation of small molecules. Anal Bioanal Chem. 2014;406(24):5897‐5907. [DOI] [PubMed] [Google Scholar]
- 83. Talebi M, Arrua RD, Gaspar A, et al. Epoxy‐based monoliths for capillary liquid chromatography of small and large molecules. Anal Bioanal Chem. 2013;405(7):2233‐2244. [DOI] [PubMed] [Google Scholar]
- 84. Bai L, Wang J, Zhang H, Liu S, Qin J, Liu H. Ionic liquid as porogen in the preparation of a polymer‐based monolith for the separation of protein by high performance liquid chromatography. Anal Methods. 2015;7(2):607‐613. [Google Scholar]
- 85. Aggarwal P, Lawson JS, Tolley HD, Lee ML. High efficiency polyethylene glycol diacrylate monoliths for reversed‐phase capillary liquid chromatography of small molecules. J Chromatogr A. 2014;1364:96‐106. [DOI] [PubMed] [Google Scholar]
- 86. Aoki H, Kubo T, Ikegami T, et al. Preparation of glycerol dimethacrylate‐based polymer monolith with unusual porous properties achieved via viscoelastic phase separation induced by monodisperse ultra high molecular weight poly(styrene) as a porogen. J Chromatogr A. 2006;1119(1‐2):66‐79. [DOI] [PubMed] [Google Scholar]
- 87. Cooper AI, Holmes AB. Synthesis of molded monolithic porous polymers using supercritical carbon dioxide as the porogenic solvent. Advanced Materials. 1999;11(15):1270‐1274. [Google Scholar]
- 88. Hebb AK, Senoo K, Cooper AI. Synthesis of porous cross‐linked polymer monoliths using 1,1,1,2‐tetrafluoroethane (R134a) as the porogen. Compos Sci Technol. 2003;63(16):2379‐2387. [Google Scholar]
- 89. Danquah MK, Forde GM. Preparation of macroporous methacrylate monolithic material with convective flow properties for bioseparation: investigating the kinetics of pore formation and hydrodynamic performance. Chem Eng J. 2008;140(1‐3):593‐599. [Google Scholar]
- 90. Desire CT, Arrua RD, Talebi M, Lacher NA, Hilder EF. Poly(ethylene glycol)‐based monolithic capillary columns for hydrophobic interaction chromatography of immunoglobulin G subclasses and variants. J Sep Sci. 2013;36(17):2782‐2792. [DOI] [PubMed] [Google Scholar]
- 91. Kornysova O, Maruska A, Owens PK, Erickson A. Non‐particulate (continuous bed or monolithic) acrylate‐based capillary columns for reversed‐phase liquid chromatography and electrochromatography. J Chromatogr A. 2005;1071(1‐2):171‐178. [DOI] [PubMed] [Google Scholar]
- 92. Li Y, Tolley HD, Lee ML. Preparation of polymer monoliths that exhibit size exclusion properties for proteins and peptides. Anal Chem. 2009;81(11):4406‐4413. [DOI] [PubMed] [Google Scholar]
- 93. Korzhikova‐Vlakh EG, Tennikova TB. Some factors affecting pore size in the synthesis of rigid polymer monoliths: theory and its applicability. J Appl Polym Sci. 2022;139(1):e51431. [Google Scholar]
- 94. Mansour FR, Arrua RD, Desire CT, Hilder EF. Non‐ionic surface active agents as additives toward a universal porogen system for porous polymer monoliths. Anal Chem. 2021;93(5):2802‐2810. [DOI] [PubMed] [Google Scholar]
- 95. Mansour FR, Desire CT, Hilder EF, Arrua RD. Effect of ethoxylated sorbitan ester surfactants on the chromatographic efficiency of poly(ethylene glycol)‐based monoliths. J Chromatogr A. 2021;1654:462464. [DOI] [PubMed] [Google Scholar]
- 96. Svec F. Less common applications of monoliths: preconcentration and solid‐phase extraction. J Chromatogr B. 2006;841(1‐2):52‐64. [DOI] [PubMed] [Google Scholar]
- 97. Arthur C, Pawliszyn J. Solid‐phase microextraction with thermal‐desorption using fused‐silica optical fibers. Anal Chem. 1990;62(19):2145‐2148. [Google Scholar]
- 98. Hu W, Zhou W, Wang C, Liu Z, Chen Z. Rapid analysis of biological samples using monolithic polymer‐based in‐tube solid‐phase microextraction with direct mass spectrometry. ACS Appl Bio Mater. 2021;4(8):6236‐6243. [DOI] [PubMed] [Google Scholar]
- 99. Wang R, Wan T, Li W, Chen Z. Schiff base network‐1 incorporated monolithic column for in‐tube solid phase microextraction of antiepileptic drugs in human plasma. Talanta. 2021;226:122098. [DOI] [PubMed] [Google Scholar]
- 100. Zhang Q, Yang Y, Zhang C, Zheng Y, Wu Y, Wang X. Development of an aptamer‐functionalized capillary monolithic column for the highly‐selective and highly‐efficient recognition of patulin. Food Control. 2021;119:107461. [Google Scholar]
- 101. Han Y, Ye Z, Chen L, Xiao L. Gold nanoparticles enumeration with dark‐field optical microscope for the sensitive glycoprotein sandwich assay. Anal Chim Acta. 2020;1109:53‐60. [DOI] [PubMed] [Google Scholar]
- 102. Wang C, Qian L, Ji L, et al. Affinity chromatography assisted comprehensive phosphoproteomics analysis of human saliva for lung cancer. Anal Chim Acta. 2020;1111:103‐113. [DOI] [PubMed] [Google Scholar]
- 103. Ali MM, Hussain D, Tang Y, et al. Boronoisophthalic acid as a novel affinity ligand for the selective capture and release of glycoproteins near physiological pH. Talanta. 2021;225:121896. [DOI] [PubMed] [Google Scholar]
- 104. Li D, Chen Y, Liu Z. Boronate affinity materials for separation and molecular recognition: structure, properties and applications. Chem Soc Rev. 2015;44(22):8097‐8123. [DOI] [PubMed] [Google Scholar]
- 105. Huang H, Zheng Q, He Y, et al. Facile synthesis of bifunctional polymer monolithic column for tunable and specific capture of glycoproteins and phosphoproteins. J Chromatogr A. 2021;1651:462329. [DOI] [PubMed] [Google Scholar]
- 106. Pichon V. Selective sample treatment using molecularly imprinted polymers. J Chromatogr A. 2007;1152(1‐2):41‐53. [DOI] [PubMed] [Google Scholar]
- 107. Moein MM. Advancements of chiral molecularly imprinted polymers in separation and sensor fields: a review of the last decade. Talanta. 2021;224:121794. [DOI] [PubMed] [Google Scholar]
- 108. Bhakta S, Mishra P. Molecularly imprinted polymer‐based sensors for cancer biomarker detection. Sens Actuator Rep. 2021;3:100061. [Google Scholar]
- 109. Nawaz N, Abu Bakar NK, Mahmud HNME, Jamaludin NS. Molecularly imprinted polymers‐based DNA biosensors. Anal Biochem. 2021;630:114328. [DOI] [PubMed] [Google Scholar]
- 110. Mostafa AM, Barton SJ, Wren SP, Barker J. Review on molecularly imprinted polymers with a focus on their application to the analysis of protein biomarkers. Trac‐Trends Anal Chem. 2021;144:116431. [Google Scholar]
- 111. Huang C, Wang H, Ma S, Bo C, Ou J, Gong B. Recent application of molecular imprinting technique in food safety. J Chromatogr A. 2021;1657:462579. [DOI] [PubMed] [Google Scholar]
- 112. Bouvarel T, Delaunay N, Pichon V. Molecularly imprinted polymers in miniaturized extraction and separation devices. J Sep Sci. 2021;44(8):1727‐1751. [DOI] [PubMed] [Google Scholar]
- 113. Szumski M, Grzywinski D, Prus W, Buszewski B. Monolithic molecularly imprinted polymeric capillary columns for isolation of aflatoxins. J Chromatogr A. 2014;1364:163‐170. [DOI] [PubMed] [Google Scholar]
- 114. Wen L, Tan X, Sun Q, Svec F, Lv Y. Smart” molecularly imprinted monoliths for the selective capture and easy release of proteins. J Sep Sci. 2016;39(16):3267‐3273. [DOI] [PubMed] [Google Scholar]
- 115. Zhang X, Zhu D, Huang C, Sun Y, Lee YI. Sensitive detection of bisphenol A in complex samples by in‐column molecularly imprinted solid‐phase extraction coupled with capillary electrophoresis. Microchem J. 2015;121:1‐5. [Google Scholar]
- 116. Bouvarel T, Chendo C, Delaunay N, Pichon V. Simplified miniaturized analytical set‐up based on molecularly imprinted polymer directly coupled to UV detection for the determination of benzoylecgonine in urine. Talanta. 2021;233:122611. [DOI] [PubMed] [Google Scholar]
- 117. Liu Y, Su Z, Wang J, Gong Z, Lyu H, Xie Z. Molecularly imprinted polymer with mixed‐mode mechanism for selective extraction and on‐line detection of ochratoxin A in beer sample. Microchem J. 2021;170:106696. [Google Scholar]
- 118. Zhou Z, Hartmann M. Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem Soc Rev. 2013;42(9):3894‐3912. [DOI] [PubMed] [Google Scholar]
- 119. Wouters B, Currivan SA, Abdulhussain N, Hankemeier T, Schoenmakers PJ. Immobilized‐enzyme reactors integrated into analytical platforms: recent advances and challenges. Trac‐Trends Anal Chem. 2021;144:116419. [Google Scholar]
- 120. Zhang H, Bai Y, Zhu N, Xu J. Microfluidic reactor with immobilized enzyme‐from construction to applications: a review. Chin J Chem Eng. 2021;30:136‐145. [Google Scholar]
- 121. Mao Y, Fan R, Li R, Ye X, Kulozik U. Flow‐through enzymatic reactors using polymer monoliths: from motivation to application. Electrophoresis. [DOI] [PubMed] [Google Scholar]
- 122. Wei ZH, Zhang X, Zhao X, Jiao YJ, Huang YP, Liu ZS. Construction of a microfluidic platform integrating online protein fractionation, denaturation, digestion, and peptide enrichment. Talanta. 2021;224:121810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable–no new data generated.


