Figure 2.
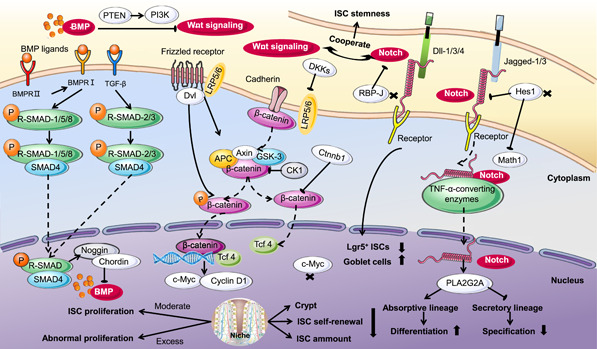
Pattern and signal transduction of ISC proliferation and differentiation. The normal operation of the ISC niche is achieved by the regulation of several signaling pathways. The Wnt, BMP, and Notch signal molecules comodulate ISC functions. Moderate activation of the Wnt signaling pathway induces ISC proliferation and self‐renewal; however, intestinal adenomas can also be caused due to abnormal proliferation resulting from excessive signaling. The BMP signaling pathway negatively regulates self‐renewal, replication, and proliferation of ISCs. Its activity is strictly controlled by cascade regulation of BMPRII, BMPRI, TGFβ, R‐SMAD, and SMAD4. Activation of the Notch signaling pathway is decisive in promoting ISC proliferation and restricting ISC specification. This determination of ISC differentiation fate is achieved by increasing absorptive lineage differentiation while inhibiting secretory lineage specification. Receptors, membrane‐bound ligands (Jagged1,3 and Delta‐like1,3,4), and DNA binding proteins constitute the main component of the Notch. TNF‐α‐converting enzymes, Hes1, Math1, and RBP‐J contribute a lot to this regulation. PTEN mediates suppression of BMP to Wnt through the PI3K signaling pathway. Meanwhile, Wnt cooperating with Notch plays an important role in maintaining ISC stemness. APC, adenomatosis polyposis coli; BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; c‐Myc, cellular Myc; Dkk1, Dickkopf‐related protein 1; Hes1, hairy and enhancer of split 1; ISC, intestinal stem cell; LRP6, lipoprotein receptor‐related protein 6; Math1, mouse atonal homolog 1; PI3K, phosphatidylinositol 3‐kinase; PTEN, phosphate and tension homology deleted on chromosome 10; RBP, recombination signal binding protein; R‐SMAD, receptor‐associated SMAD; SMAD4, drosophila mothers against decapentaplegic protein; TGFβ, transforming growth factor β; TNF‐α, tumor necrosis factor alpha.
