Fe3O4@SiO2-NH-GA-[(CH2)4-SO3H]3 catalyzed the synthesis of acridine-1,8-dionesa.

| ||
|---|---|---|

|
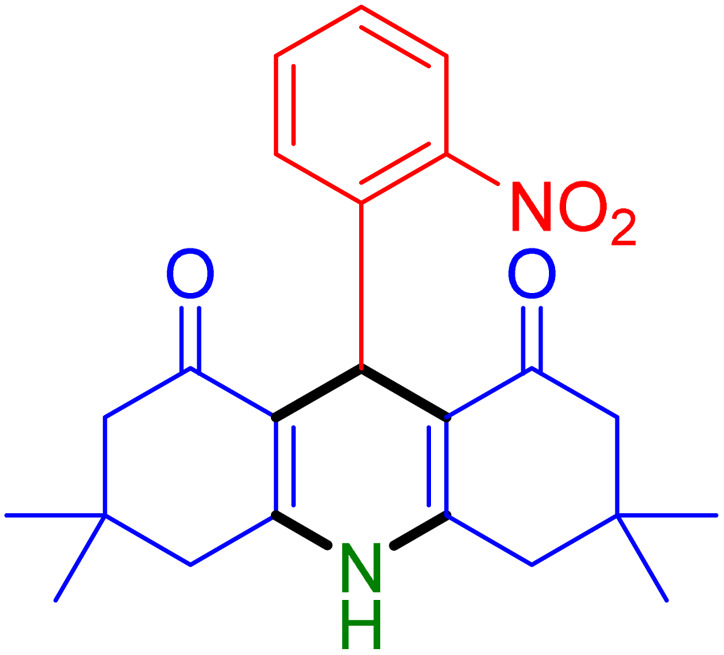
|

|
| Entry 1: 4a, 90%b | Entry 2: 4b, 88% | Entry 3: 4c, 94% |

|

|

|
| Entry 4: 4d, 96% | Entry 5: 4e, 85% | Entry 6: 4f, 87% |
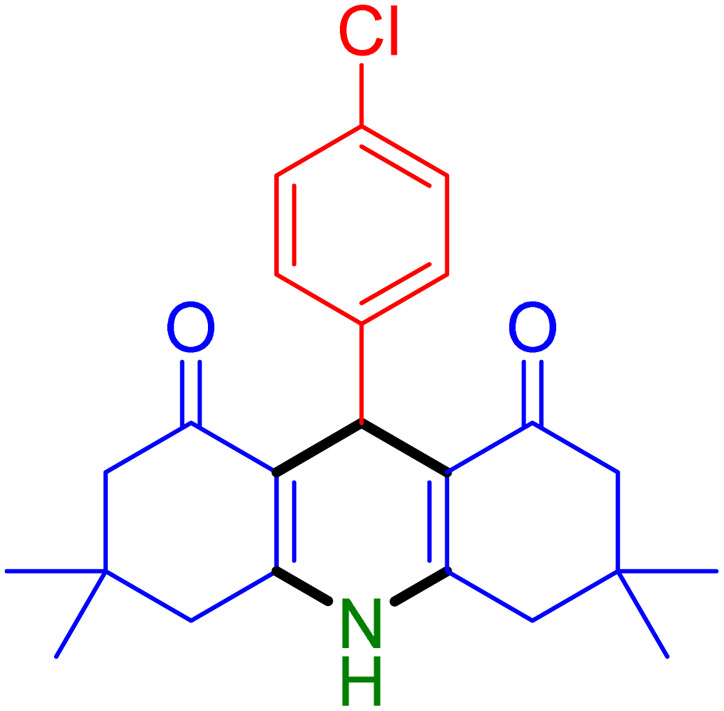
|

|
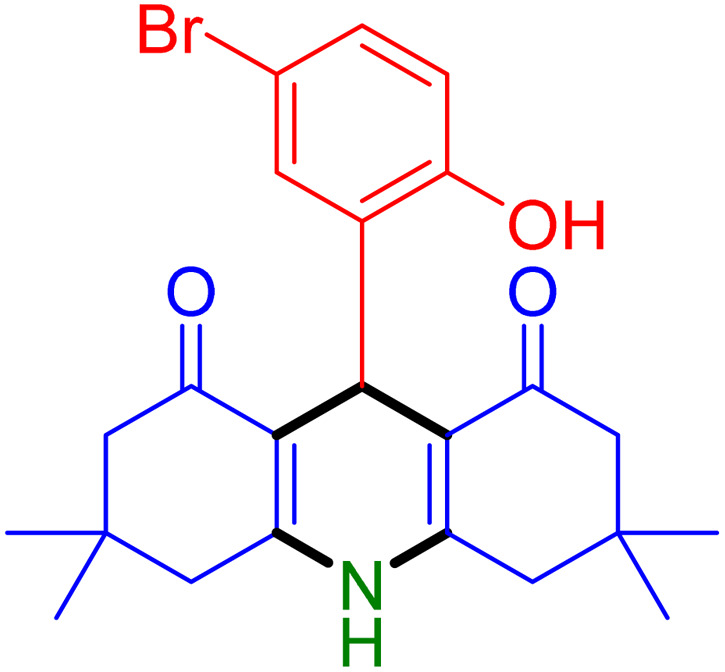
|
| Entry 7: 4g, 87% | Entry 8: 4h, 81% | Entry 9: 4i, 80% |

|
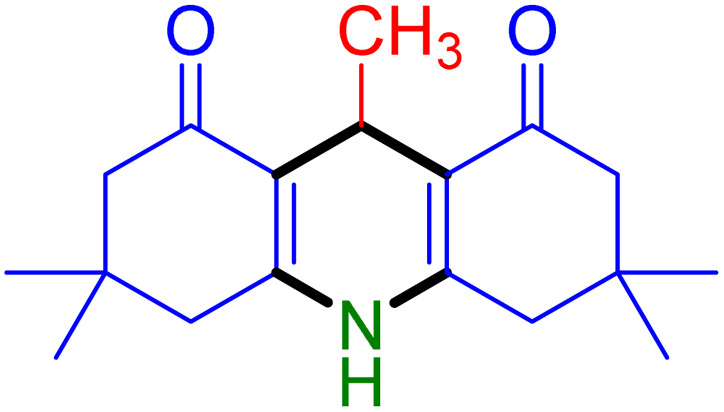
|

|
| Entry 10: 4j, 80% | Entry 11: 4k, 74%c | Entry 12: 4l, 70%c |

|
|

|
| Entry 13: 4m, 83% | Entry 14: 4n, 84% | Entry 15: 4o, 84% |

|

|

|
| Entry 16: 4p, 87% | Entry 17: 4q, 73% | Entry 18: 4r, 77% |

|

|

|
| Entry 19: 4s, 78% | Entry 20: 4t, 94% | Entry 21: 4u, 96% |

|

|
|
| Entry 22: 4v, 92% | Entry 23: 4w, 94% | |
Experimental conditions: aldehyde (1.0 mmol), dimedone (2.0 mmol), and ammonium acetate/amines (1.1 mmol), catalyst (5.0 mg), in water (2.0 mL) at 60 °C.
Isolated yield.
The reaction was performed in a sealed tube.
