Abstract
Aging is inevitable, but the lifespan (duration of life) and healthspan (healthy aging) vary greatly among individuals and across species. Unlocking the secrets behind these differences has captivated scientific curiosity for ages. This review presents relevant recent advances in genetics and cell biology that are shedding new light by untangling how subtle changes in conserved genes, pathways, and epigenetic factors influence organismal senescence and associated declines.
Biogerontology is a complex and rapidly growing field aimed at elucidating genetic modifications that extend lifespan and healthspan. This review explores gerontogenes, genes influencing lifespan and healthspan across species. Though subtle differences exist, long-lived individuals such as centenarians demonstrate extended healthspans, and numerous studies confirm the heritability of longevity/healthspan genes. Importantly, genes and gerontogenes are directly and indirectly involved in DNA repair, insulin/IGF-1 and mTOR signaling pathways, long non-coding RNAs, sirtuins, and heat shock proteins. The complex interactions between genetics and epigenetics are teased apart. While more research into optimizing healthspan is needed, conserved gerontogenes offer synergistic potential to forestall aging and age-related diseases. Understanding complex longevity genetics brings closer the goal of extending not only lifespan but quality years of life.
The primary aim of human Biogerontology is to enhance lifespan and healthspan, but the question remains: are current genetic modifications effectively promoting healthy aging? This article collates the advancements in gerontogenes that enhance lifespan and improve healthspan alongside their potential challenges.
Keywords: gerontology, gerontogenes, longevity, healthspan, lifespan
Introduction and background
The field of Biogerontology (biology of aging) is a complex and rapidly growing discipline. Genes that influence lifespan are broadly referred to as longevity genes, while all genes studied in Biogerontology are collectively known as gerontogenes. In ancient Greek and Roman times, demographic studies generally indicate a life expectancy between 20 and 35 years [1,2]. Despite the limitations of these earlier sources, it can be inferred that very few individuals during this time lived beyond 90 years [3]. While this outcome is considered low by 21st-century standards, it is noteworthy that individuals of the Greco-Roman era were less afflicted by aging-related diseases such as cancer, cardiovascular disorders, and multiorgan failures. This fact contradicts the idealized portrayal of lifespan extension, challenging the assumption that a longer lifespan naturally equates to a healthier life. The 21st century is ideally the era of aging. The world is getting older, and the numbers are consistently rising, with nearly 22% of the world population being ≥60 years old by the year 2050 [4]. Predictably, the cost of healthcare will rise due to numerous aging-associated diseases with immeasurable effects on quality of life. With most illnesses indicating underlying physiologic changes, delaying aging may help curb multiple health complications concurrently via modulating genetic pathways. As the mystery of aging becomes increasingly deciphered, the study of Biogerontology is founded on three key principles. First, lifespan is defined as the age at which death occurs, and the essential lifespan of a species marks the point beyond which there is a consistent decline in functional and physiological capabilities due to cellular aging, culminating in death [5,6]. The onset of aging is considered to begin after the essential lifespan is surpassed. Second, there is no single biological pathway or set of aging-associated genes (gerontogenes) identified as the sole cause of aging and death [7-9]. Lastly, the rate of aging varies among different species, individuals, and even within different organs and tissues of a single organism. This suggests that co-dependence and interconnected biological pathways across all levels play a crucial role in determining the overall lifespan of an individual [8,10,11].
Longevity and healthspan are often used interchangeably, yet they encompass distinct aspects. Longevity primarily centers around lifespan, the observed duration of an organism’s life [12], whereas healthspan focuses on healthy aging. Healthspan extends beyond the presence or absence of diseases or disease-susceptible alleles. It includes the development and maintenance of physical, physiological, and psychological capabilities [13] that contribute to overall well-being and delayed onset of age-associated diseases. Research indicates that individuals who live exceptionally long lives, such as centenarians, often experience a prolonged healthspan with minimal incidences of aging-related diseases such as cancer, cardiovascular disease, dementia, hypertension, and Alzheimer’s disease [14]. Healthy aging encompasses old age and good health with commendable body performance levels such as mobility and unharmed cognition [15]. Highlighting the significance of longevity and healthy aging genes is crucial, as numerous studies have demonstrated their heritability [15]. Notably, the likelihood of inheriting these genes increases with age, showing a probability of 0.48 in men and 0.33 in women, particularly in centenarians aged between 100 and 109 years [16]. Further evidence of the association between inherited longevity genes and increased age has been observed in extensive studies involving over 20,000 Scandinavian twins [17], Icelanders [18], and other centenarian populations [19,20]. Additionally, comprehensive studies like the New England Centenarian Study [21], the Leiden Longevity Study [22], and the Long Life Family Study [23] have compared the offspring of long-lived individuals to contemporaneous controls. These offspring not only exhibited longer average lifespans but also displayed various healthy aging characteristics, including beneficial lipid profiles [24], a low rate of cardiovascular and metabolic diseases [25], and a lower prevalence of hypertension compared to their age-matched controls [24,26]. In recent decades, Biogerontology has undergone a significant transformation, shifting from limited access to aging pathways to advanced genetic modifications that can extend lifespan. Contemporary Biogerontology focuses on how these longevity genes impact healthspan. It is noteworthy that healthspan can be significantly altered, either enhanced or deteriorated, due to interventions targeting lifespan, often leading to increased morbidity [26].
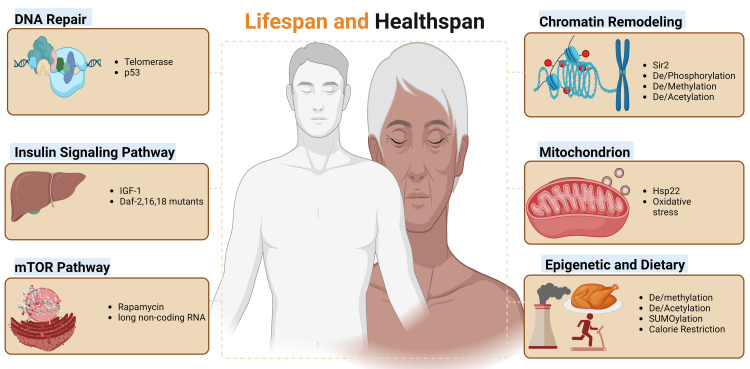
The relationship between an organism’s lifespan and the maintenance of cellular youthfulness reveals a complex interconnection of pathways. While there is no single, definite hypothesis to explain the rate of aging, identified contributing factors include genetics, a combination of epigenetics, physiological factors, and lifestyle choices. Four broad groups of genes are conserved across various kingdoms and have been identified to influence aging. These include genes responsible for (i) DNA repair enzymes, (ii) proteins in the insulin signaling pathway, (iii) mechanistic target of rapamycin (mTOR) signaling pathway proteins (translation regulators), and (iv) chromatin remodeling enzymes. Further studies extensively explore the role of mitochondrial heat shock protein genes, such as Hsp22, and various epigenetic and environmental factors that contribute to aging (Figure 1).
Figure 1. Genetic, epigenetic, and environmental factors influencing lifespan and healthspan.
Image generated using BioRender.com.
Review
DNA repair enzymes
Possessing efficient DNA repair enzymes resistant to mutations enhances longevity by mitigating the effects of aging-related “wear and tear.” The wear-and-tear hypothesis proposes that the efficiency of cell division and enzymatic functions decline with age [27,28]. Mutations in DNA repair enzymes, even in younger individuals, can lead to premature aging conditions, such as progerias [29,30]. The transcription factor p53 plays a critical role in regulating cell division. It arrests the cell cycle and activates DNA repair enzymes [31,32]. The pleiotropic effect of p53 allows multi-level regulation from diverse factors, demonstrated with mouse double minute 2 (Mdm2) and Mdm4 [33]. In the absence or damage to telomeres, p53 becomes active, halting DNA replication and facilitating DNA repair. The role of telomeres and telomerase activity in cellular immortality has been a long-standing proposition [34]. The telomerase enzyme replenishes telomere length after each cell cycle. Studies have shown that telomere lengths are longer in children than adults, supporting telomeres’ role in aging [34]. Again, a study using transgenic mice, replacing mouse telomerase RNA (mTR) with neomycin-resistant genes decreased telomere length and telomerase activity with age [35,36]. Similarly, older zebrafish exhibited shorter telomeric repeats (TTAGGG)n in the terminal restriction fragment (TRF) compared to their younger counterparts [37]. Reduction in the telomeric repeats (TTAGGG)n sequence has been associated with chromosomal instability, increased aneuploidy, and a higher incidence of cell-to-cell fusions [38]. Similarly, cells harboring a mouse telomerase null mutation exhibited no telomerase activity and, consequently, the least lifespan [39]. The length of telomeric repeats which cap chromosomal ends is inversely proportional to aging. Premature aging disorders, such as progeria, spleen atrophy, disrupted germinal centers, and reduced proliferation in bone marrow and neural stem cells, have been associated with a lack of telomerase and a deleted or nonfunctional telomerase gene [40,41]. This association led to the hypothesis that extending telomeric repeats might enhance healthspan. However, attempts to maintain longer telomeric stability present challenges, as they often seed tumorigenesis due to uncontrolled cellular division [42]. Present methods for measuring telomere length, including TRF analysis and quantitative polymerase chain reaction, are subject to minor margins of error that affect the accuracy of telomere length results. Despite the experimental and logistical reliability of these established techniques, their inherent drawbacks can be addressed by more advanced methodologies such as single telomere length analysis and telomere shortest length assay (TESLA), albeit at increased financial and labor costs [43]. Telomere length exhibits variations of approximately ±2-4% per month, challenging the prevailing assumption of consistent telomeric attrition with age [44].
Mechanistic target of rapamycin pathway
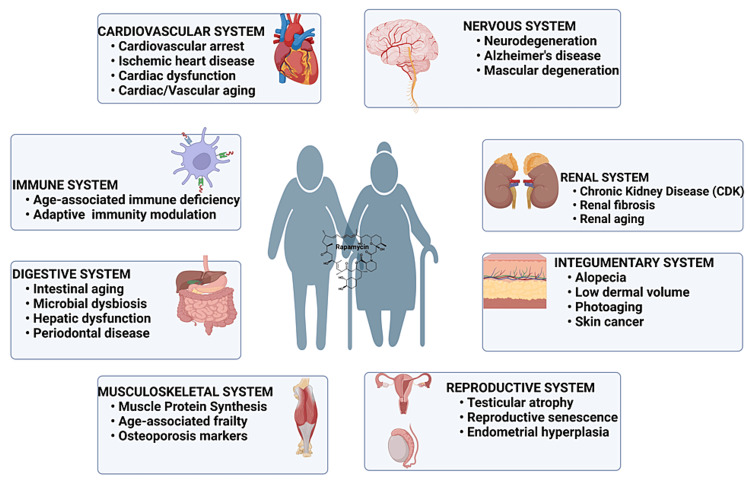
Altering genes in the mTORC1 pathway directly impacts cellular longevity [45]. When activated, the insulin signaling pathway upregulates the activity of the mTORC1 pathway and inhibits the FoxO transcription factor [45,46]. In the mTORC1 pathway, the protein kinase complex mTORC1 stimulates the conversion of mRNA into essential proteins and enzymes in response to nutrients and hormones [47]. Inhibiting mTORC1 interferes with insulin signal transmission, leading to reduced metabolism, slowed aging, and a decline in age-related conditions such as cognitive dysfunction [48,49]. Since the discovery of rapamycin as an antibiotic in 1975, it has been extensively studied for its potential to extend lifespan [50]. Studies show that rapamycin, in combination with FK506-binding proteins, downregulates genes in the mTOR pathway and is evident in yeast [51], nematodes [52], rotifers [53], drosophila [54], and mouse models [55,56]. Interestingly, rapamycin has a sexually dimorphic effect on mTOR signaling with varying mean healthspan extension in females than males in different research models [52,57,58]. Growing evidence indicates that rapamycin contributes to an improved healthspan, as seen through delayed aging of organs (Figure 2) and reduced incidence of disease conditions, including liver degeneration, ovarian aging, heart abnormalities, and benign tumors in the adrenal gland [59,60]. Mechanistically, rapamycin improves healthspan via inhibition of the mTOR pathway, which impacts multiple biological processes such as apoptosis, inflammation, cell growth, and metabolism [60,61]. Rapamycin specifically inhibits the mTORC1 signaling pathway [62] to enhance healthspan through delayed aging in kidneys, liver, ovaries, intestines, respiratory, circulatory, integumentary, and immune systems. A major setback in rapamycin clinical administration is the simultaneous inhibition of mTORC2 signaling while blocking the mTORC1 pathway [62]. This off-target effect induces adverse effects on metabolic and immunologic functions and can be prevented by selectively inhibiting mTORC1 with rapamycin derivatives and structural analogs. An extensive study introduced the importance of long noncoding RNAs as essential regulators of mTOR signaling, in general, to improve lifespan expectancy and decline onset of aging-related disorders and cancer in humans [63]. Currently, rapamycin is a great candidate for both lifespan and healthspan extension with extensive research and clinical studies.
Figure 2. General role of rapamycin in sustaining organ health.
Image generated using BioRender.com.
Insulin and insulin-like growth factor 1 signaling pathways
Similar to DNA repair enzymes, genes within the insulin signaling pathway exhibit a functionally conserved role in aging across various species. Downregulation of these genes indicates limited food availability, leading to a decline in metabolism and an enhanced synthesis of free radical scavenging enzymes via the upregulation of the FoxO/DAF-16 transcription factor [64-66]. FoxO is crucial to the immortality of hydra and a conserved longevity regulator operating downstream of insulin signaling pathways of several species in the animal kingdom. Several reports indicate that the IGF-1 receptor regulates lifespan and oxidative stress in mice [67,68]. Upon modification of different exons in the IGF-1 gene and feeding mice ad libitum, heterozygous IGF-1 mice lived longer than the wild type [69,70]. In animal models, such as Caenorhabditis elegans and Drosophila, reduced insulin signaling has been linked to an extended lifespan [71,72]. As aging broadly depicts a decline in physical activities, studies involving the maximum velocity of C. elegans revealed that the daf-2 mutant (E1370) exhibited a higher maximum velocity, approximately twice that of the wild type [73]. Notably, the daf-2 mutant/insulin/IGF-1 receptor exhibits pleiotropic effects by exponentially enhancing lifespan and immunity while diminishing reproduction and motility [74]. Loss-of-function mutation in daf-18(nr2037), encoding phosphatase and tensin homolog (PTEN), is essential to the extended lifespan of daf-2 mutants [75]. PTEN dephosphorylates phosphatidylinositol 3,4,5-trisphosphate to phosphatidylinositol 4,5-bisphosphate [76]. Despite disorders in insulin signaling causing conditions such as type 1 and 2 diabetes and neuronal dysfunction due to metabolic stress [77], they have been associated with extended lifespans in humans [78], C. elegans [64], and Drosophila melanogaster [79]. A shared trait among these organisms is the reduction in serum IGF-1, associated with the insulin/IGF signaling cascade. However, mutagens in ingested food and free radicals such as hydroxyl and superoxide radicals formed as metabolic by-products accumulate to induce age-related diseases. Reduced IGF-1 signaling by deleting the IGF-1 receptor decreases metabolism and eventually reduces the overall cell senescence caused by oxidative stress [77]. Furthermore, due to higher metabolic rates in males than females, the overall impact of lifespan extension varies between genders [80]. This conservation of insulin signaling pathways and their influence on aging highlights a fundamental aspect of biology that transcends species, offering potential insights for human aging and longevity research.
Chromatin remodeling
Genes encoding proteins to modify chromatin structure influence aging and aging-associated diseases by regulating the spatial expression of genes during development. Chromatin modification techniques include but are not limited to methylation/demethylation, phosphorylation, acetylation/deacetylation, and sumoylation. Geneticist Amar Klar first described sirtuins in the 1970s when he discovered the silent information regulators II (Sir2) gene in Saccharomyces cerevisiae cells. However, the only study demonstrating an effect on lifestyle was published 20 years later in 1991 by Leonard P. Guarante [81]. Sirtuins belong to the class of deacetylases that help coordinate DNA repair, cell survival, healthy aging, development, apoptosis, and metabolic control [82]. The sirtuin gene, known for its anti-aging properties and encoding proteins involved in chromatin silencing through histone deacetylation, is found across the eukaryotic kingdoms [83]. As growth occurs, sirtuin gene products, such as enzymes and proteins, are re-purposed to repair single- and double-stranded DNA breaks at the expense of chromatin silencing [82]. Consequently, genes that were initially silenced become active as aging progresses. Cognitive decline, associated with the mammalian aging syndrome, can be mitigated by upregulating DNA methylation and histone (H4K12) acetylation in the brain’s frontal lobes [83]. Sirtuins comprise seven regulatory proteins for metabolism, antioxidant protection, and cell cycle regulation. Deletion of the Sir2 gene, responsible for the expression of sirtuins, shortens the lifespan of S. cerevisiae and has, therefore, become an important focus of research on the aging process [84]. Contemporary methodologies associated with chromatin analysis are both high-throughput and labor-intensive, necessitating the use of chromatin immunoprecipitation, antibody staining, microscopy, and, at times, sequencing. The experimental workflows employed in chromatin studies can introduce background noise and variability and often permit only relative quantification [85].
In conjunction with sirtuins, polyphenols such as butein, fisetin, and resveratrol have been shown to extend yeast lifespan by 31%, 55%, and 70%, respectively, also contributing to an increase in the maximum lifespan for each of these activators [86]. Research has highlighted the potency of resveratrol to delay the onset of aging-associated diseases by mimicking calorie restriction [87]. Over time, the homologous recombination between the ribosomal DNA repeats, forming a circular, autonomously replicating extrachromosomal DNA, which is toxic in old cells. Resveratrol increases the activity of yeast Sir2 to prevent the formation of extrachromosomal structures [88]. The synergistic increment in lifespan in the presence of polyphenols is a culmination of antioxidant, metal ion chelating, and free radical scavenging roles of polyphenols and the chromatin silencing effect of sirtuin via histone deacetylation. Invariably, no observable increments in lifespan are noticed in Sir2 null mutants in S. cerevisiae [89] and humans [90]. Hence, Sir2 primarily regulates longevity, while polyphenols directly stimulate Sir2 activity in vivo.
Epigenetics and diet
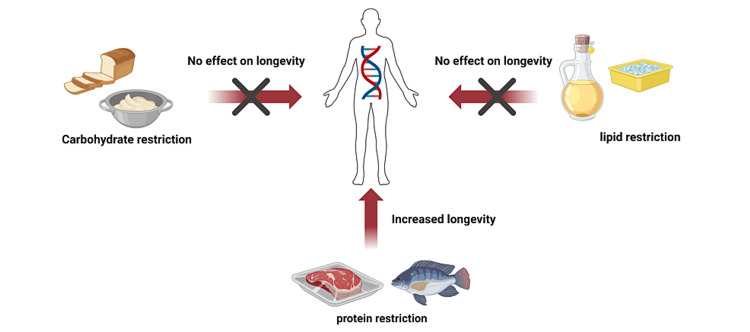
In addition to genetic determinants, the effects of epigenetic factors influence the overall lifespan of an organism. Susceptibility to errors during cell division cycles and possible activation of oncogenes increase with prolonged cell divisions, making aging a protective mechanism against tumorigenesis [91]. The methylation of CpG islands occurs under several conditions such as genetic, dietary, and exposure to xenobiotics and mutagens. While Ames dwarf mice have thrice fewer CpG islands than wildtype, caloric restriction prevents demethylation at hypomethylated regions and enhances methylation of hypermethylated loci than rapamycin [92]. Thus, the epigenetic influence on the aging process is far more convoluted and is species-specific. Therefore, studies involving cell longevity and healthspan involve cautious consideration of the numerous ongoing, interrelated pathways, cell divisions, and reactions. Dietary intake has strong correlations with aging. A highly inflammatory diet dwindles the body’s repair potential and eventually speeds up aging [93]. This reduction in the repair potential arises from the continuous disruption of cell types responsible for the repair of inflammation resulting from tissue injury [94]. Dietary restriction (caloric reduction) is known to delay aging and the onset of diseases as well as enhance longevity in most organisms [95]. Dietary enhancement of longevity is best achieved by a combination of low protein/high carbohydrate food rations (Figure 3) [96] as low protein/high carbohydrates inhibit the mTORC1 pathway, enhance thrombospondin signaling pathway, and suppress the production of reactive oxygen species by the mitochondrion as opposed to the lower intake of carbohydrates and fats [97]. This reduction in reactive oxygen species coupled with a decrease in metabolic rate has long been regarded as the means through which dietary restriction may prolong lifespan. Several studies have cited caloric reduction as important in preventing the signs of aging, such as telomere deterioration, epigenetic changes, genome instability, mitochondrion inefficiency, and cellular senescence [42,98,99]. Moreover, caloric reduction effectively limits age-associated alterations in DNA methylations in vital organs [100] and delays age-related decline in mitochondrial biogenesis and function [101].
Figure 3. Effects of dietary restriction of major food nutrients on longevity.
Image generated using BioRender.com.
Mitochondrion and aging
Aging is generally associated with increased oxidative stress and declining mitochondrial efficiency. The mitochondrial gene encoding heat shock proteins, Hsp, has garnered significant interest in anti-aging studies. A study by Lagunas-Rangel highlighted the influence of G protein-coupled receptors on lifespan using animal and human models [102]. Given mitochondria’s sensitivity to reactive oxygen species, the presence of genes that produce antioxidants within mitochondria is essential. The overexpression of Hsp22 has been reported to protect mitochondrial proteins within motoneurons and extend the lifespan of D. melanogaster [103]. Hsp22 genes contribute to extending the lifespan in D. melanogaster by predominantly guarding against oxidative stress. Studies have highlighted the functional roles of mitochondrial Hsp22 in lifespan extension and oxidative stress resistance in Drosophila [104]. Conversely, overexpression of Hsp22 yielded a reduced lifespan in mouse models due to myocardial hypertrophy [105]. An optimal mitochondrial function stems from good protein quality. The Hsp22 proteins act as molecular chaperones against ruined proteins, as unchecked damaged proteins gradually amass into toxic aggregates with further deteriorating effects on mitochondrial integrity. Hsp22 concentration is an aging biomarker in D. melanogaster and predicts the remaining lifespan. Ultimately, Hsp22 increases longevity by chaperoning damaged proteins and improving stress resistance.
Conclusions
To enhance the applicability of these models to human research, efforts should concentrate on identifying study metrics that accurately represent the unique physiological and pathological aspects of aging in different species. The gerontogenes encoding telomerase/telomeric repeats, insulin-like growth factor receptor 1, Sir2, and mitochondrial heat shock proteins (Hsp22) enhance longevity, while daf-2 gene mutants and downregulation of mTOR signaling genes by rapamycin enhance both longevity and healthspan. Healthspan is a complex, multi-metric analysis of functional and physiologic efficiencies of organs in different organisms. More studies are needed to streamline the healthspan conception and the best multi-metrics for a specific anti-aging model organism. Moreover, these conserved gerontogenes influencing longevity and healthspan are functionally nonexclusive; hence, understanding the synergistic efforts of two or more gerontogenes can introduce novel techniques to minimize geriatric syndrome while maintaining healthy cells.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Paa Kwesi Ankrah, Enock D. Mensah, Kwabena Dabie, Caleb Mensah, Benjamin Akangbe, Jonathan Essuman
Acquisition, analysis, or interpretation of data: Paa Kwesi Ankrah
Drafting of the manuscript: Paa Kwesi Ankrah, Enock D. Mensah, Kwabena Dabie, Caleb Mensah, Benjamin Akangbe, Jonathan Essuman
Critical review of the manuscript for important intellectual content: Paa Kwesi Ankrah, Enock D. Mensah, Kwabena Dabie, Caleb Mensah, Benjamin Akangbe, Jonathan Essuman
Supervision: Paa Kwesi Ankrah
References
- 1.Scheidel W. The Cambridge Economic History of the Greco-Roman World. Cambridge, UK: Cambridge University Press; 2007. Demography; pp. 38–86. [Google Scholar]
- 2.Hoppa RD, Vaupel JW. Paleodemography: Age Distributions From Skeletal Samples. Cambridge, UK: Cambridge University Press; 2002. The Rostock manifesto for paleodemography: the way from stage to age; pp. 1–8. [Google Scholar]
- 3.Evolution in health and medicine Sackler colloquium: evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Finch CE. Proc Natl Acad Sci U S A. 2010;107 Suppl 1:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ageing Ageing. World Health Organization. Ageing. World Health organization. [ Jan; 2024 ]. 2018. https://www.who.int/news-room/facts-in-pictures/detail/ageing#:~:text=The%20world's%20population%20is%20rapidly,of%20the%20total%20global%20population) https://www.who.int/news-room/facts-in-pictures/detail/ageing#:~:text=The%20world's%20population%20is%20rapidly,of%20the%20total%20global%20population)
- 5.Biogerontology: the next step. Rattan SI. Ann N Y Acad Sci. 2000;908:282–290. doi: 10.1111/j.1749-6632.2000.tb06655.x. [DOI] [PubMed] [Google Scholar]
- 6.Understanding and modulating ageing. Rattan SI, Clark BF. IUBMB Life. 2005;57:297–304. doi: 10.1080/15216540500092195. [DOI] [PubMed] [Google Scholar]
- 7.Gerontogenes: real or virtual? Rattan SI. FASEB J. 1995;9:284–286. doi: 10.1096/fasebj.9.2.7781932. [DOI] [PubMed] [Google Scholar]
- 8.Finch CE, Kirkwood TBL. Chance, Development, and Aging. New. New York, NY: Oxford University Press; 2000. Chance, Development, and Aging; p. 0. [Google Scholar]
- 9.Longevity mutants do not establish any "new science" of ageing. Holliday R, Rattan SI. Biogerontology. 2010;11:507–511. doi: 10.1007/s10522-010-9288-1. [DOI] [PubMed] [Google Scholar]
- 10.Biogerontology: from here to where? The Lord Cohen Medal Lecture-2011. Rattan SI. Biogerontology. 2012;13:83–91. doi: 10.1007/s10522-011-9354-3. [DOI] [PubMed] [Google Scholar]
- 11.Rattan SI. Molecular Basis of Nutrition and Aging. London: Academic Press; 2016. Molecular and cellular basis of aging; pp. 3–9. [Google Scholar]
- 12.What is lifespan regulation and why does it exist? Carnes BA. Biogerontology. 2011;12:367–374. doi: 10.1007/s10522-011-9338-3. [DOI] [PubMed] [Google Scholar]
- 13.From lifespan to healthspan: the role of nutrition in healthy ageing. Wickramasinghe K, Mathers JC, Wopereis S, Marsman DS, Griffiths JC. J Nutr Sci. 2020;9:0. doi: 10.1017/jns.2020.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longo V. New York, NY: Penguin; 2019. The Longevity Diet: Slow Aging, Fight Disease, Optimize Weight. [Google Scholar]
- 15.Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The genetics of extreme longevity: lessons from the new England centenarian study. Sebastiani P, Perls TT. Front Genet. 2012;3:277. doi: 10.3389/fgene.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genetic influence on human lifespan and longevity. vB Hjelmborg J, Iachine I, Skytthe A, et al. https://books.google.com/books?hl=en&lr=&id=_QJE-fiKUP8C&oi=fnd&pg=PA217&dq=McGue+M,+Koskenvuo+J,+Kaprio+NL,+Pedersen+K,+Pedersen+NL&ots=wLPla9olvg&sig=7WVqAr4ddCq7cDdhqj67yu3KOvE#v=onepage&q=McGue%20M%2C%20Koskenvuo%20J%2C%20Kaprio%20NL%2C%20Pedersen%20K%2C%20Pedersen%20NL&f=false. Hum Genet. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 18.Inheritance of human longevity in Iceland. Gudmundsson H, Gudbjartsson DF, Frigge M, Gulcher JR, Stefánsson K. Eur J Hum Genet. 2000;8:743–749. doi: 10.1038/sj.ejhg.5200527. [DOI] [PubMed] [Google Scholar]
- 19.Morbidity profiles of centenarians: survivors, delayers, and escapers. Evert J, Lawler E, Bogan H, Perls T. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 20.Heritability and validity of healthy physical aging (wellness) in elderly male twins. Reed T, Dick DM. Twin Res. 2003;6:227–234. doi: 10.1375/136905203765693889. [DOI] [PubMed] [Google Scholar]
- 21.Lower all-cause, cardiovascular, and cancer mortality in centenarians' offspring. Terry DF, Wilcox MA, McCormick MA, Pennington JY, Schoenhofen EA, Andersen SL, Perls TT. J Am Geriatr Soc. 2004;52:2074–2076. doi: 10.1111/j.1532-5415.2004.52561.x. [DOI] [PubMed] [Google Scholar]
- 22.Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Schoenmaker M, de Craen AJ, de Meijer PH, Beekman M, Blauw GJ, Slagboom PE, Westendorp RG. Eur J Hum Genet. 2006;14:79–84. doi: 10.1038/sj.ejhg.5201508. [DOI] [PubMed] [Google Scholar]
- 23.Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Newman AB, Glynn NW, Taylor CA, et al. Aging (Albany NY) 2011;3:63–76. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipid metabolism in long-lived families: the Leiden Longevity Study. Vaarhorst AA, Beekman M, Suchiman EH, et al. Age (Dordr) 2011;33:219–227. doi: 10.1007/s11357-010-9172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. Westendorp RG, van Heemst D, Rozing MP, et al. J Am Geriatr Soc. 2009;57:1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 26.Renal function in familial longevity: the Leiden Longevity Study. de Goeij MC, Halbesma N, Dekker FW, et al. Exp Gerontol. 2014;51:65–70. doi: 10.1016/j.exger.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 27.No time to age: uncoupling aging from chronological time. Larocca D, Lee J, West MD, Labat I, Sternberg H. Genes (Basel) 2021;12:611. doi: 10.3390/genes12050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passos JF, Kirkwood TB. Textbook of Geriatric Dentistry. West Sussex, UK: John Wiley and Sons Ltd.; 2015. Biological and physiological aspects of aging; pp. 29–36. [Google Scholar]
- 29.The central role of DNA damage in the ageing process. Schumacher B, Pothof J, Vijg J, Hoeijmakers JH. Nature. 2021;592:695–703. doi: 10.1038/s41586-021-03307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Principles of the molecular and cellular mechanisms of aging. da Silva PF, Schumacher B. J Invest Dermatol. 2021;141:951–960. doi: 10.1016/j.jid.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Exploring the multiple roles of guardian of the genome: P53. Feroz W, Sheikh AMA. Egyptian J Med Hum Genet. 2020;21:1–23. [Google Scholar]
- 32.Telomere length, telomeric proteins and genomic instability during the multistep carcinogenic process. Raynaud CM, Sabatier L, Philipot O, Olaussen KA, Soria JC. Crit Rev Oncol Hematol. 2008;66:99–117. doi: 10.1016/j.critrevonc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Kumari R, Jat P. Front Cell Dev Biol. 2021;9:645593. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newborn telomere length predicts later life telomere length: tracking telomere length from birth to child- and adulthood. Martens DS, Van Der Stukken C, Derom C, Thiery E, Bijnens EM, Nawrot TS. EBioMedicine. 2021;63:103164. doi: 10.1016/j.ebiom.2020.103164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Defects in mTR stability and telomerase activity produced by the Dkc1 A353V mutation in dyskeratosis congenita are rescued by a peptide from the dyskerin TruB domain. Machado-Pinilla R, Carrillo J, Manguan-Garcia C, et al. Clin Transl Oncol. 2012;14:755–763. doi: 10.1007/s12094-012-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 37.Behaviour of telomere and telomerase during aging and regeneration in zebrafish. Anchelin M, Murcia L, Alcaraz-Pérez F, García-Navarro EM, Cayuela ML. PLoS One. 2011;6:0. doi: 10.1371/journal.pone.0016955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escape from cellular senescence is associated with chromosomal instability in oral pre-malignancy. Prime SS, Cirillo N, Parkinson EK. Biology (Basel) 2023;12:103. doi: 10.3390/biology12010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Full length RTEL1 is required for the elongation of the single-stranded telomeric overhang by telomerase. Awad A, Glousker G, Lamm N, et al. Nucleic Acids Res. 2020;48:7239–7251. doi: 10.1093/nar/gkaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallmarks of aging: an expanding universe. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Wu T, Fu F, Cheng J, et al. Ovarian Aging. Singapore: Springer Nature Singapore; 2023. The cellular and molecular mechanisms of ovarian aging; pp. 119–169. [Google Scholar]
- 42.Targeting telomerase for cancer therapy. Guterres AN, Villanueva J. Oncogene. 2020;39:5811–5824. doi: 10.1038/s41388-020-01405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comparison of telomere length measurement methods. Lai TP, Wright WE, Shay JW. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160451. doi: 10.1098/rstb.2016.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blood cell telomere length is a dynamic feature. Svenson U, Nordfjäll K, Baird D, Roger L, Osterman P, Hellenius ML, Roos G. PLoS One. 2011;6:0. doi: 10.1371/journal.pone.0021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Chen CC, Jeon SM, Bhaskar PT, et al. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Therapeutic strategies targeting FOXO transcription factors. Calissi G, Lam EW, Link W. Nat Rev Drug Discov. 2021;20:21–38. doi: 10.1038/s41573-020-0088-2. [DOI] [PubMed] [Google Scholar]
- 47.Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Lamming DW, Ye L, Katajisto P, et al. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.mTOR at the nexus of nutrition, growth, ageing and disease. Liu GY, Sabatini DM. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antioxidant modulation of mTOR and sirtuin pathways in age-related neurodegenerative diseases. Abdullah A, Mohd Murshid N, Makpol S. Mol Neurobiol. 2020;57:5193–5207. doi: 10.1007/s12035-020-02083-1. [DOI] [PubMed] [Google Scholar]
- 50.Rapamycin: an inhibiTOR of aging emerges from the soil of Easter Island. Arriola Apelo SI, Lamming DW. J Gerontol A Biol Sci Med Sci. 2016;71:841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Transcriptional and epigenetic regulation by the mechanistic target of rapamycin complex 1 pathway. Laribee RN. J Mol Biol. 2018;430:4874–4890. doi: 10.1016/j.jmb.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sex-specific tradeoffs with growth and fitness following life-span extension by rapamycin in an outcrossing nematode, Caenorhabditis remanei. Lind MI, Zwoinska MK, Meurling S, Carlsson H, Maklakov AA. J Gerontol A Biol Sci Med Sci. 2016;71:882–890. doi: 10.1093/gerona/glv174. [DOI] [PubMed] [Google Scholar]
- 53.Effects of rapamycin on life span and on expression of TOR and S6K in Brachionus calyciflorus (Rotifera) Xu H, Liu L, Su Y, et al. https://www.int-res.com/abstracts/ab/v26/p49-56/ Aquat Biol. 2017;26:49–56. [Google Scholar]
- 54.Dissecting the biology of mTORC1 beyond rapamycin. Yang G, Francis D, Krycer JR, et al. Sci Signal. 2021;14:0. doi: 10.1126/scisignal.abe0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A novel rapamycin analog is highly selective for mTORC1 in vivo. Schreiber KH, Arriola Apelo SI, Yu D, et al. Nat Commun. 2019;10:3194. doi: 10.1038/s41467-019-11174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inactivation of regulatory-associated protein of mTOR (Raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. Dai Q, Xie F, Han Y, et al. J Biol Chem. 2017;292:196–204. doi: 10.1074/jbc.M116.764761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Baar EL, Carbajal KA, Ong IM, Lamming DW. Aging Cell. 2016;15:155–166. doi: 10.1111/acel.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sexual dimorphism in liver cell cycle and senescence signalling pathways in young and old rats. Lomas-Soria C, Cox LA, Nathanielsz PW, Zambrano E. J Physiol. 2021;599:4309–4320. doi: 10.1113/JP281822. [DOI] [PubMed] [Google Scholar]
- 59.Therapeutic interventions for aging: the case of cellular senescence. Soto-Gamez A, Demaria M. Drug Discov Today. 2017;22:786–795. doi: 10.1016/j.drudis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 60.The role of rapamycin in healthspan extension via the delay of organ aging. Zhang Y, Zhang J, Wang S. Ageing Res Rev. 2021;70:101376. doi: 10.1016/j.arr.2021.101376. [DOI] [PubMed] [Google Scholar]
- 61.Rapamycin improves healthspan but not inflammaging in nfκb1(-/-) mice. Correia-Melo C, Birch J, Fielder E, et al. Aging Cell. 2019;18:0. doi: 10.1111/acel.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inhibition of the mechanistic target of rapamycin (mTOR)-rapamycin and beyond. Lamming DW. Cold Spring Harb Perspect Med. 2016;6:0. doi: 10.1101/cshperspect.a025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Panwar V, Singh A, Bhatt M, et al. Signal Transduct Target Ther. 2023;8:375. doi: 10.1038/s41392-023-01608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.FOXO transcription factors as mediators of stress adaptation. Rodriguez-Colman MJ, Dansen TB, Burgering BM. Nat Rev Mol Cell Biol. 2024;25:46–64. doi: 10.1038/s41580-023-00649-0. [DOI] [PubMed] [Google Scholar]
- 65.Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans. Wang Y, Shi J, Liu K, Wang Y, Xu Y, Liu Y. Food Sci. 2023;12:1391–1401. [Google Scholar]
- 66.Oxidative stress and life span determination in the nematode Caenorhabditis elegans. Honda Y, Honda S. Ann N Y Acad Sci. 2002;959:466–474. doi: 10.1111/j.1749-6632.2002.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 67.The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. Altintas O, Park S, Lee SJ. BMB Rep. 2016;49:81–92. doi: 10.5483/BMBRep.2016.49.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.C. elegans SIRT6/7 homolog SIR-2.4 promotes DAF-16 relocalization and function during stress. Chiang WC, Tishkoff DX, Yang B, et al. PLoS Genet. 2012;8:0. doi: 10.1371/journal.pgen.1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Extending lifespan by modulating the growth hormone/insulin-like growth factor-1 axis: coming of age. Duran-Ortiz S, List EO, Basu R, Kopchick JJ. Pituitary. 2021;24:438–456. doi: 10.1007/s11102-020-01117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Holzenberger M, Dupont J, Ducos B, et al. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 71.An overview of the insulin signaling pathway in model organisms Drosophila melanogaster and Caenorhabditis elegans. Biglou SG, Bendena WG, Chin-Sang I. Peptides. 2021;145:170640. doi: 10.1016/j.peptides.2021.170640. [DOI] [PubMed] [Google Scholar]
- 72.Insulin signaling regulates longevity through protein phosphorylation in Caenorhabditis elegans. Li WJ, Wang CW, Tao L, et al. Nat Commun. 2021;12:4568. doi: 10.1038/s41467-021-24816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Hahm JH, Kim S, DiLoreto R, Shi C, Lee SJ, Murphy CT, Nam HG. Nat Commun. 2015;6:8919. doi: 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling. Park HH, Hwang W, Ham S, et al. Nat Commun. 2021;12:5631. doi: 10.1038/s41467-021-25920-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.PTEN regulates PI(3,4)P(2) signaling downstream of class I PI3K. Malek M, Kielkowska A, Chessa T, et al. https://www.cell.com/molecular-cell/pdf/S1097-2765(17)30702-5.pdf. Mol Cell. 2017;68:566–580. doi: 10.1016/j.molcel.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim B, Sims-Robinson C, Sakowski SA, Feldman EA. Neurobiology of Brain Disorders. New York, NY: Academic Press; 2023. Diabetes and cognitive dysfunction; pp. 185–201. [Google Scholar]
- 77.Insulin signaling and life span. Avogaro A, de Kreutzenberg SV, Fadini GP. Pflugers Arch. 2010;459:301–314. doi: 10.1007/s00424-009-0721-8. [DOI] [PubMed] [Google Scholar]
- 78.The phosphatase CSW controls life span by insulin signaling and metabolism throughout adult life in Drosophila. Ruzzi LR, Schilman PE, San Martin A, Lew SE, Gelb BD, Pagani MR. Front Genet. 2020;11:364. doi: 10.3389/fgene.2020.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dzene E. London, UK: Queen Mary University of London; 2022. The Role of Intermediary Metabolism in Skin Ageing and Interplay With Senescence. [Google Scholar]
- 80.Sex differences in lifespan. Austad SN, Fischer KE. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Why is longevity still a scientific mystery? Sirtuins-past, present and future. Ziętara P, Dziewięcka M, Augustyniak M. Int J Mol Sci. 2022;24:728. doi: 10.3390/ijms24010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moniot S, You W, Steegborn C. Introductory Review on Sirtuins in Biology, Aging, and Disease. New York, NY: Academic Press; 2018. Structural and mechanistic insights in sirtuin catalysis and pharmacological modulation; pp. 63–70. [Google Scholar]
- 83.Histone acetylation modifiers in the pathogenesis of Alzheimer's disease. Lu X, Wang L, Yu C, Yu D, Yu G. Front Cell Neurosci. 2015;9:226. doi: 10.3389/fncel.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antiaging agents: safe interventions to slow aging and healthy life span extension. Liu JK. Nat Prod Bioprospect. 2022;12:18. doi: 10.1007/s13659-022-00339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biohorology and biomarkers of aging: current state-of-the-art, challenges and opportunities. Galkin F, Mamoshina P, Aliper A, de Magalhães JP, Gladyshev VN, Zhavoronkov A. Ageing Res Rev. 2020;60:101050. doi: 10.1016/j.arr.2020.101050. [DOI] [PubMed] [Google Scholar]
- 86.Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Howitz KT, Bitterman KJ, Cohen HY, et al. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 87.The significance of caloric restriction mimetics as anti-aging drugs. Nassar K, El-Mekawey D, Elmasry AE, Refaey MS, El-Sayed Ghoneim M, Elshaier YA. Biochem Biophys Res Commun. 2024;692:149354. doi: 10.1016/j.bbrc.2023.149354. [DOI] [PubMed] [Google Scholar]
- 88.Sirtuin signaling in cellular senescence and aging. Lee SH, Lee JH, Lee HY, Min KJ. BMB Rep. 2019;52:24–34. doi: 10.5483/BMBRep.2019.52.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hilsabeck TA. San Antonio, TX: The University of Texas at San Antonio; 2016. Using Systems Biology to Predict Lifespan in the Yeast Saccharomyces cerevisiae. [Google Scholar]
- 90.Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 91.DNA damage-how and why we age? Yousefzadeh M, Henpita C, Vyas R, Soto-Palma C, Robbins P, Niedernhofer L. Elife. 2021;10:0. doi: 10.7554/eLife.62852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Cole JJ, Robertson NA, Rather MI, et al. Genome Biol. 2017;18:58. doi: 10.1186/s13059-017-1185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diet and inflammation: possible effects on immunity, chronic diseases, and life span. Ricordi C, Garcia-Contreras M, Farnetti S. J Am Coll Nutr. 2015;34 Suppl 1:10–13. doi: 10.1080/07315724.2015.1080101. [DOI] [PubMed] [Google Scholar]
- 94.Role of fatty acids and polyphenols in inflammatory gene transcription and their impact on obesity, metabolic syndrome and diabetes. Sears B, Ricordi C. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=cc68e580dbec56621903598db60c631b8be5ab0d. Eur Rev Med Pharmacol Sci. 2012;16:1137–1154. [PubMed] [Google Scholar]
- 95.The impact of low-protein high-carbohydrate diets on aging and lifespan. Le Couteur DG, Solon-Biet S, Cogger VC, et al. Cell Mol Life Sci. 2016;73:1237–1252. doi: 10.1007/s00018-015-2120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The Geometric Framework for Nutrition as a tool in precision medicine. Simpson SJ, Le Couteur DG, James DE, George J, Gunton JE, Solon-Biet SM, Raubenheimer D. Nutr Healthy Aging. 2017;4:217–226. doi: 10.3233/NHA-170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.The impact of dietary protein intake on longevity and metabolic health. Kitada M, Ogura Y, Monno I, Koya D. EBioMedicine. 2019;43:632–640. doi: 10.1016/j.ebiom.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, Li J. Signal Transduct Target Ther. 2022;7:391. doi: 10.1038/s41392-022-01251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Does diet influence aging? Evidence from animal studies [in press] Le Couteur DG, Raubenheimer D, Solon-Biet S, de Cabo R, Simpson SJ. J Intern Med. 2022 doi: 10.1111/joim.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.The impact of caloric restriction on the epigenetic signatures of aging. Gensous N, Franceschi C, Santoro A, Milazzo M, Garagnani P, Bacalini MG. Int J Mol Sci. 2019;20:2022. doi: 10.3390/ijms20082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.The age-sensitive efficacy of calorie restriction on mitochondrial biogenesis and mtDNA damage in rat liver. Chimienti G, Picca A, Fracasso F, et al. Int J Mol Sci. 2021;22:1665. doi: 10.3390/ijms22041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.G protein-coupled receptors that influence lifespan of human and animal models. Lagunas-Rangel FA. Biogerontology. 2022;23:1–19. doi: 10.1007/s10522-021-09945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Age-dependent expression profiles of two adaptogenic systems and thermotolerance in Drosophila melanogaster. Shilova V, Zatsepina O, Zakluta A, Karpov D, Chuvakova L, Garbuz D, Evgen'ev M. Cell Stress Chaperones. 2020;25:305–315. doi: 10.1007/s12192-020-01074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drosophila melanogaster mitochondrial Hsp22: a role in resistance to oxidative stress, aging and the mitochondrial unfolding protein response. Morrow G, Le Pécheur M, Tanguay RM. Biogerontology. 2016;17:61–70. doi: 10.1007/s10522-015-9591-y. [DOI] [PubMed] [Google Scholar]
- 105.Hsp22 overexpression induces myocardial hypertrophy, senescence and reduced life span through enhanced oxidative stress. Morin D, Long R, Panel M, et al. Free Radic Biol Med. 2019;137:194–200. doi: 10.1016/j.freeradbiomed.2019.04.035. [DOI] [PubMed] [Google Scholar]