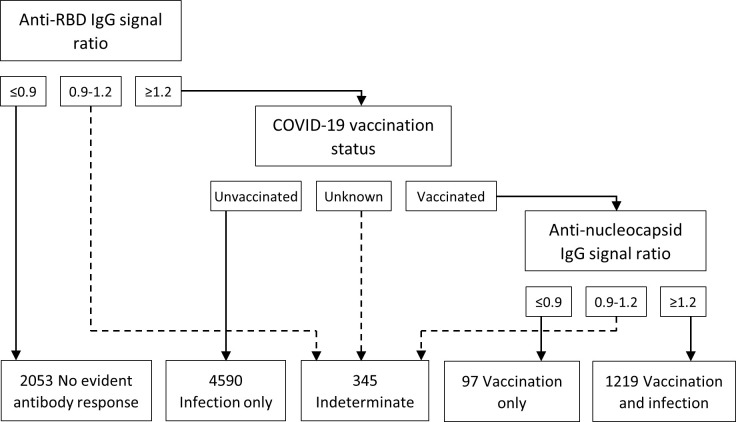
Fig 2. Antibody response categorization based on anti-RBD IgG, anti-nucleocapsid IgG, and COVID-19 vaccination status (N = 8,304).
The Tetracore FlexImmArray SARS-CoV-2 Human IgG Antibody Test used is a multiplex bead assay with three SARS-CoV-2 targets (i.e., nucleocapsid, receptor-binding domain (RBD) of spike, and fusion) to facilitate distinguishing between infection only, vaccination only, and combined vaccination and infection antibody responses when considered with vaccination history. Participants who reported receiving a Sinopharm or unknown vaccine were categorized as indeterminate due to the inability to distinguish between vaccination only and hybrid vaccination and infection antibody responses from inactivated or attenuated virus vaccines. Adapted from Duarte et al. 2021.