Abstract
Meconium aspiration syndrome (MAS) is a clinical condition characterized by respiratory distress in neonates born through meconium-stained amniotic fluid (MSAF). Despite advances in obstetric practices and perinatal care, MAS remains an important cause of morbidity and mortality in term and post-term newborns. Since the 1960s, there have been significant changes in the perinatal and postnatal management of infants born through MSAF. Routine endotracheal suctioning is no longer recommended in both vigorous and non-vigorous neonates with MSAF. Supportive care along with new treatments such as surfactant, inhaled nitric oxide, and high-frequency ventilation has significantly improved the outcome of MAS patients. However, determining the most appropriate approach for this condition continues to be a topic of debate. This review offers an updated overview of the epidemiology, etiopathogenesis, diagnosis, management, and prognosis of infants with MAS.
Keywords: meconium aspiration syndrome (MAS), meconium-stained amniotic fluid (MSAF), newborn, respiratory distress syndrome (RDS)
Introduction
Highlights
Despite advancements in obstetrical and neonatal care, meconium aspiration syndrome (MAS) remains an important source of morbidity and mortality in term and post-term newborns.
The lung injury caused by meconium is complex and can be attributed to a variety of factors, including mechanical obstruction of the airways, inactivation of surfactant, and chemical pneumonitis.
Treatment strategies for MAS comprise oxygen supplementation, surfactant therapy, and supportive care. The use of steroid therapy remains a subject of debate.
Among preventive strategies, elective induction of labour for pregnancies greater than or equal to 41 weeks has been associated with a significant reduction in the incidence of MAS.
Meconium aspiration syndrome (MAS) is a major cause of morbidity and mortality among neonates, particularly in developing countries1. Over the past four decades, the concepts of the pathophysiology and management of MAS have undergone tremendous change. In the past, routine intrapartum and postnatal endotracheal suctioning of meconium was recommended for both vigorous and non-vigorous infants born through meconium-stained amniotic fluid (MSAF) to prevent MAS. However, this practice has been re-evaluated with recent research suggesting that routine suctioning may not be beneficial, and is no longer recommended2,3. The spectrum of manifestations associated with meconium aspiration is broad, ranging from mild distress to more severe respiratory failure4. By synthesizing current knowledge and research findings, this review aims to provide an updated overview of the epidemiology, etiopathogenesis, diagnosis, management, and prognosis of infants with MAS.
Definition
MAS is defined as respiratory distress in a neonate born through MSAF with characteristic radiological findings (hyperinflation and patchy opacities) whose symptoms cannot be explained otherwise5. According to Cleary and Wiswell6, the severity of MAS can be defined as mild (FiO2 <0.40 for less than 48 h), moderate (FiO2 >0.40 for more than 48 h without air leak), or severe (mechanical ventilation for more than 48 h and/or pulmonary hypertension).
Epidemiology
MAS primarily affects near-term, term, or post-term newborns, as meconium is uncommon in amniotic fluid before 34 weeks of gestation. MSAF is observed in ~4–22% of all births7, with higher rates (up to 23–52%) seen in pregnancies beyond 42 weeks’ gestation8,9. However, only a small percentage (3–12%) of infants born through MSAF develop MAS10,11. The risk of MAS is increased in black Americans, Africans, and Pacific Islanders6,12. There is an increased risk of MAS in IUGR infants13,14. Already compromised by placental insufficiency and chronic intrauterine hypoxia, they are more prone to asphyxia than normally grown infants.
Among infants with MAS, around 20% are non-vigorous at birth, and approximately one-third require intubation and mechanical ventilation for respiratory support11. The mortality rate for MAS is estimated to be between 5 and 12%15. Overall, MAS accounts for about 10% of cases of neonatal respiratory failure16. In developed countries, the incidence of MAS has shown a decline due to improvements in obstetric practices and perinatal care17.
Pathophysiology of MAS
Meconium is a viscous, dark green substance composed of swallowed amniotic fluid, lanugo, and gastrointestinal secretions. Normally, the passage of meconium from the foetus into the amnion is prevented by the lack of intestinal peristalsis due to low motilin levels, and the tonic contraction of the anal sphincter18. The exact reason why some infants pass meconium in utero is not fully understood. According to Poggi and Ghidini19, foetal hypoxic stress induces relaxation of the anal sphincter and stimulates gastrointestinal peristalsis, leading to meconium passage. Foetal hypoxia also may stimulate gasping movements that result in meconium aspiration. When aspirated, meconium can affect the lungs through three mechanisms: airway obstruction, inflammation, and surfactant dysfunction3 (Fig. 1).
Figure 1.
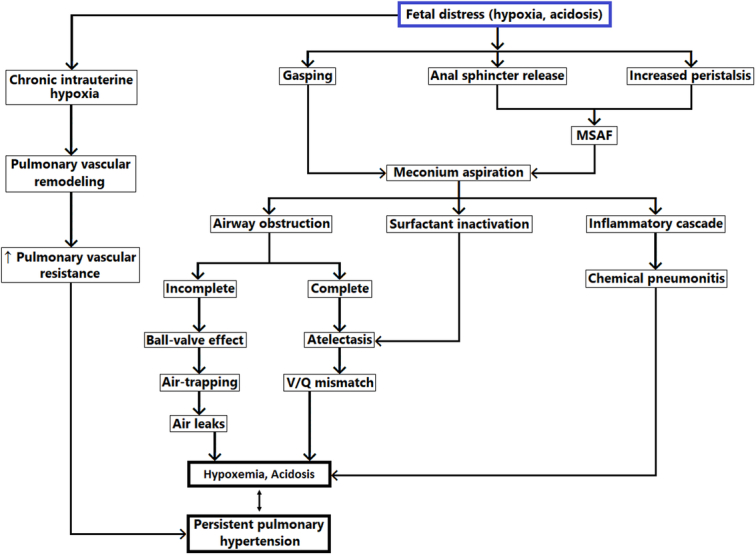
Pathophysiology of meconium aspiration syndrome. Legend: MSAF, meconium-stained amniotic fluid; V/Q mismatch, ventilation/perfusion mismatch.
Airway obstruction
Airway obstruction after intrapartum ingestion of meconium was considered the primary mechanism of MAS in the past20. Meconium, when aspirated into the trachea and bronchi, can lead to airway obstruction. If the obstruction is complete, the airways and alveoli beyond the blockage collapse, resulting in post-obstructive atelectasis and ventilation-perfusion mismatch. In cases of partial obstruction, air may pass beyond the obstruction during inspiration but become trapped during expiration, causing a “ball-valve” effect. This can lead to hyperinflation in certain areas of the lungs and may contribute to the development of pulmonary air leaks. This pattern of hyperinflation surrounded by atelectasis can appear as a “salt and pepper” appearance on chest X-rays.
Inflammation
Aspiration of meconium initiates an inflammatory response in the lungs. Meconium is a good chemoattractant for neutrophils21. A few hours after meconium aspiration, neutrophils and macrophages are found in the alveoli and lung parenchyma. Meconium is also a source of proinflammatory mediators such as tumour necrosis factor-alpha (TNFα), interleukin-1 β (IL-1β), IL-6, and IL-8. Phospholipase A2, present in meconium, can damage alveolar cells by releasing arachidonic acid from cell membranes.
Apoptosis, cellular infiltration, and increased concentrations of inflammatory cytokines in bronchoalveolar lavage of animal lungs instilled with meconium have been demonstrated in numerous studies22,23.
Surfactant dysfunction
Meconium present in the alveoli can inactivate the endogenous surfactant and decrease the synthesis of surfactant proteins A and B24. This results in lung atelectasis and can increase ventilation-perfusion mismatch. The precise mechanisms by which meconium inactivates pulmonary surfactant remain uncertain. However, several components of meconium, especially free fatty acids, cholesterol, and bile acids, are known to impair lung function25,26.
Persistent pulmonary hypertension
Persistent pulmonary hypertension of the newborn (PPHN) is common in neonates with MAS. Indeed, a majority of cases of PPHN are associated with MAS27. Both acute pulmonary arterial vasoconstriction and abnormal muscularization of the intra-acinous arteries play crucial roles in the pathophysiology of PPHN. Pulmonary arterial vasoconstriction can be induced by hypoxia as a result of the mechanisms discussed earlier (mechanical obstruction, chemical inflammation, or surfactant inactivation). Chronic hypoxia can also lead to PPHN through the development of abnormal pulmonary arterial muscularization28. This histologic finding indicates a chronic alteration likely occurring before birth, rather than being a response to acute meconium aspiration. Therefore, meconium passage associated with PPHN can act as both a direct pathogenic cause of lung damage and a potential indicator of chronic intrauterine hypoxia.
Clinical features
The first and most apparent sign is the presence of MSAF at birth in a non-vigorous newborn, which suggests that the infant may have experienced asphyxia in utero. A newborn with MAS typically exhibits signs of respiratory distress of varying severity, including tachypnea, cyanosis, nasal flaring, and respiratory retractions5. Affected infants typically have a barrel-shaped chest with an increased anterior-posterior diameter caused by overinflation. On auscultation, widespread crackles may be heard in the lungs. General findings of MAS may also include conditions related to hypoxia such as encephalopathy, heart failure, poor peripheral circulation, and reduced urine output.
In severe cases, newborns with MAS can enter a harmful cycle: hypoxaemia leads to acidosis, and both of these conditions contribute to worsening pulmonary hypertension. Pulmonary hypertension can lead to a right-to-left shunt through the foramen ovale and the ductus arteriosus, worsening hypoxaemia and perpetuating the cycle.
Diagnosis
The following criteria have been suggested for the diagnosis of MAS29:
Respiratory distress in a neonate born through MSAF.
Requirement for supplemental oxygen to maintain haemoglobin oxygen saturation greater than 92%.
Requirement for supplemental oxygen beginning before 2 h of life and lasting at least 12 h.
Absence of congenital anomalies of the airways, lungs, or heart.
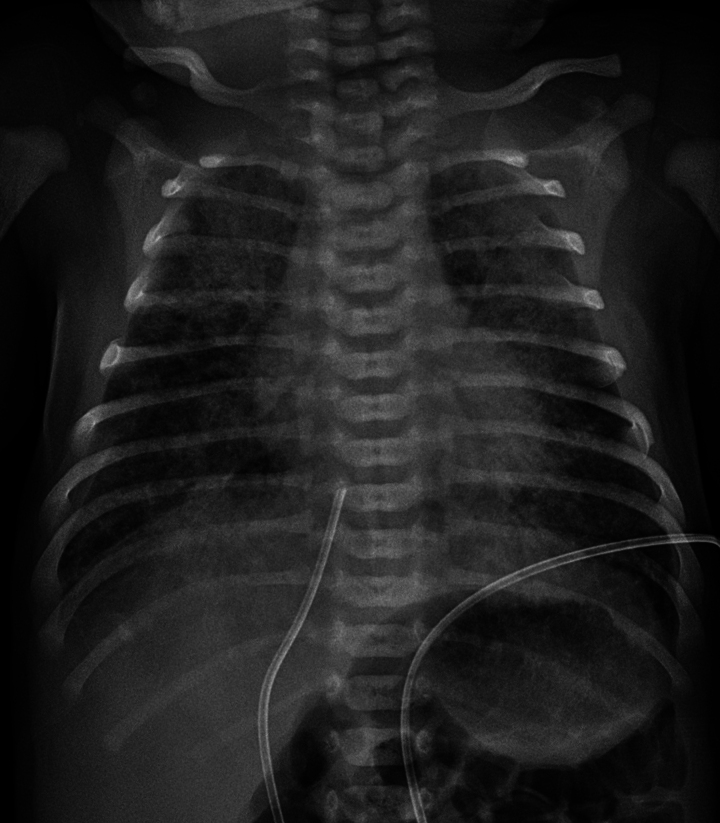
The initial chest X-ray may reveal streaky linear densities progressing to hyperinflation with diffuse patchy densities (Fig. 2). Air leak occurs in 10–30% of infants with MAS. Arterial blood gas measurements typically show hypoxaemia and hypercarbia.
Figure 2.
Chest radiography of a full-term infant with meconium aspiration.
Lung ultrasound has emerged as a valuable diagnostic and prognostic tool in the NICU for evaluating MAS30. A few lung ultrasound signs, including B-lines, consolidations, atelectasis, and bronchograms, have been observed in MAS patients, indicating the potential use of this technique in clinical practice31.
Echocardiography is the gold standard to confirm the diagnosis of PPHN. The key features of PPHN include abnormal right ventricular dilatation, tricuspid regurgitation (in ~60–85% of patients with PPHN), and right-to-left shunting through the ductus arteriosus and/or foramen ovale32,33.
The differential diagnosis of MAS includes other causes of respiratory distress. These may include transient tachypnea of the newborn (occurs in infants born between 34 and 37 weeks of gestation and typically resolves within 24 h), sepsis, pneumonia, and congenital heart disease.
Treatment of meconium aspiration syndrome
Supportive care
All infants with MAS should be admitted to a level III NICU and monitored using pulse oximetry. One of the key aspects of managing infants with MAS is to minimize their exposure to stress and discomfort, which can exacerbate hypoxia and contribute to right-to-left shunting. Maintaining normothermia, and correcting acidosis, hypoglycemia, and other metabolic disorders are also crucial (Fig. 3).
Figure 3.
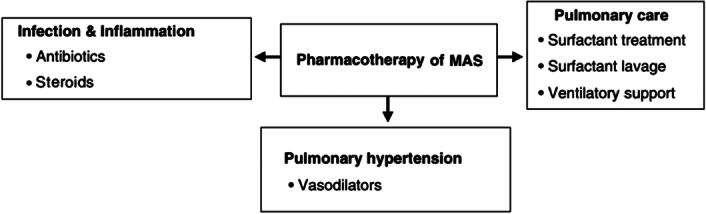
Pharmacotherapy of MAS. MAS, meconium aspiration syndrome.
Nasogastric aspiration
Prophylactic nasogastric suctioning before the first feed in infants born through MSAF has been suggested to reduce the risk of feed intolerance and secondary meconium aspiration. Several randomized trials have found no clear benefit of this practice in reducing both outcomes34–37. However, a recent meta-analysis of six trials by Deshmukh et al.38 found that prophylactic lavage might reduce the risk of feed intolerance in meconium-stained neonates by ~30%. Despite these findings, the authors have interpreted their results with caution due to several limitations of the study, including the lack of a standard definition for feed intolerance, subjectivity in the assessment of outcomes, and a lack of data on potential confounding factors such as sepsis and swallowed maternal blood. To adequately assess the role of this procedure in preventing secondary meconium aspiration, a large randomized controlled trial is needed.
Respiratory support
Oxygen administration is the primary treatment for MAS, and in many mild cases, it may be the only therapy needed. Oxygen saturations should be targeted within the range of 90–95%. Depending on the severity, respiratory support may vary. Some infants may require only oxygen by hood. Nasal continuous positive airway pressure (nCPAP) has proven superior in reducing the need for mechanical ventilation compared to hood oxygen39.
Approximately 40% of newborns with MAS require intubation and mechanical ventilation. The aim of mechanical ventilation should be to improve oxygenation and simultaneously minimize barotrauma. Indications for intubation include high oxygen requirement (FiO2 >0.8), respiratory acidosis (persistent arterial pH <7.25), pulmonary hypertension, and circulatory instability40. The ideal ventilation strategy is still a subject of debate due to the lack of clinical trials providing definitive guidelines. This is further complicated by the complex pathophysiology of MAS, which includes areas of atelectasis alongside overinflation, ventilation-perfusion mismatch, and airway compromise.
In cases with marked regional or global atelectasis, high peak inspiratory pressure (PIP) (up to a maximum of 30 cm H2O), and high positive-end expiratory pressure (PEEP) (4–7 cm H2O) with longer inspiratory time are utilized to facilitate alveolar recruitment41.
In infants with associated PPHN, it is generally advised to consider higher rates of ventilation (50–70 breaths per minute) along with higher FiO2 levels (80–100%) to maintain PaO2 between 70 and 100 mm Hg and PaCO2 between 35 and 45 mm Hg40,41.
Surfactant
Surfactant inhibition is one of the mechanisms of injury in MAS. The Canadian Pediatric Society recommends exogenous surfactant therapy for all intubated infants with MAS requiring FiO2 greater than or equal to 50%42. Surfactant may be administered as bolus therapy or bronchoalveolar lavage43.
Meta-analysis of four randomized controlled trials (RCTs) showed a reduction in the severity of respiratory illness and a decrease in the number of infants with progressive respiratory failure requiring ECMO44. However, there was no significant difference in mortality, length of ventilation, duration of oxygen use, pneumothorax, pulmonary interstitial emphysema, or chronic lung disease.
Antibiotics
Meconium provides the perfect medium for bacterial overgrowth. Empiric antibiotic treatment is commonly initiated in infants with MAS while awaiting culture results. However, the evidence supporting the routine use of antibiotics in MAS is limited, and studies have not shown clear advantages in terms of reducing the incidence of sepsis, mortality rates, or the duration of hospital stay45–47. The role of antibiotics in the management of MAS may need to be re-evaluated in well-designed trials.
Corticosteroids
Pulmonary and systemic inflammation are key features of MAS pathophysiology, providing the rationale behind the use of steroids in cases of severe MAS. According to a meta-analysis of nine RCTs48, steroids did not reduce in-hospital mortality among infants with MAS nor had any effect on the length of hospital stay and duration of oxygen support.
In a few animal studies, inhaled budesonide has shown a promising response when instilled intratracheally alone or with surfactant49,50. Some studies conducted in India suggested that steroid therapy might decrease the duration of oxygen therapy and the length of hospital stay51,52. Currently, there is no conclusive evidence to propose routine steroid therapy in the management of MAS.
Inhaled nitric oxide
PPHN is a frequent complication and a leading cause of death in severe cases of MAS20. Inhaled nitric oxide (iNO) acts on vascular smooth muscle, causing selective pulmonary vasodilation in ventilated areas of the lung53. This makes it an ideal agent for PPHN, as it improves oxygenation by reducing the ventilation-perfusion mismatch.
Randomized controlled trials have indicated that iNO therapy can decrease the need for ECMO and reduce mortality in full-term and near-term neonates with hypoxic respiratory failure and PPHN54,55. An oxygenation index of 25 may be considered the optimal time to initiate iNO therapy56.
Combining high-frequency ventilation (HFV) and iNO can also be beneficial. Better lung inflation during HFV can decrease intrapulmonary shunting and improve iNO delivery to the pulmonary circulation, thus enhancing the response to iNO. This synergistic combination has been observed to improve oxygenation in some infants with severe PPHN.
A significant proportion of infants with PPHN (30–50%) may not respond to iNO therapy. Additionally, the set-up for iNO involves high costs and is not readily available in developing countries. Other pulmonary vasodilators, including sildenafil, milrinone, and magnesium sulfate, have been tried in newborns, but the number treated is few, with no large control studies available to date57.
Extracorporeal membrane oxygenation (ECMO)
In developed countries, the usage of ECMO for MAS has significantly decreased due to the availability of iNO and HFV. However, for severe and refractory hypoxaemia associated with MAS, ECMO may still be utilized as a final rescue therapy. Infants with MAS constitute ~35% of the infant population who require ECMO58. When treated with ECMO, infants with MAS have a high survival rate approaching almost 95%59.
Therapeutic hypothermia
Whole-body hypothermia is the cornerstone treatment of neonatal hypoxic-ischaemic encephalopathy resulting from perinatal asphyxia60. MAS patients should be offered therapeutic hypothermia when the criteria for asphyxia are met61.
Prognosis
The use of new treatments such as surfactant, nitric oxide, HFV, and ECMO has significantly improved the overall prognosis of infants with MAS. A retrospective study of full-term infants diagnosed with MAS from 1997 to 2007 revealed a mortality rate of 1.2%62. This is a considerable decrease compared to the previously reported mortality rate of 4.2% from 1973 to 198763. In the same study, an APGAR score <3, the requirement for ventilator support within 48 h, repeated use of vasopressor agents, and the administration of cefotaxime were identified as independent risk factors for mortality62. However, while the survival rates have improved over the years, the long-term morbidity among survivors remains a significant concern64. Infants requiring prolonged mechanical ventilation may develop bronchopulmonary dysplasia. The current data indicate that there is an increased prevalence of asthmatic symptoms and abnormal bronchial reactivity among survivors of the MAS65. The neurological outcome is more dismal, with ~21% of infants developing cerebral palsy or global developmental delay66.
Prevention
Prevention plays a crucial role in managing MAS due to its potential for poor outcomes. The following obstetric practices have been identified as potential measures to reduce the incidence of MAS.
Amnioinfusion
Amnioinfusion using an isotonic sterile solution (normal saline or ringer lactate) has been proposed to reduce the risk of MAS by diluting the meconium, thus reducing its mechanical and inflammatory effects. The additional fluid can also potentially cushion the umbilical cord, helping to prevent recurrent umbilical compressions that can lead to foetal acidemia.
A meta-analysis of RCTs conducted by Pierce et al.67 found that intrapartum amnioinfusion was significantly associated with a reduced risk of MAS, meconium below the vocal cords, and neonatal acidemia. However, a more recent Cochrane meta-analysis68, which separated the studies based on clinical settings, found that amnioinfusion only reduced the risk of MAS in settings with limited peripartum surveillance but not in clinical settings with standard peripartum surveillance. Given these findings, the American College of Obstetricians and Gynecologists concluded that routine prophylactic amnioinfusion for dilution of MSAF is not recommended for the prevention of MAS69.
Prevention of post-term pregnancy
The risk of MAS is highest in post-term deliveries (≥42 weeks). Therefore, preventing prolonged pregnancy by labour induction might reduce the risk of MAS. Elective induction of labour at 41 weeks of gestation is a reasonable approach. Meta-analysis of 14 randomized controlled trials suggests that elective induction of labour for pregnancies more than or equal to 41 weeks is associated with a significant reduction in the incidence of MAS70.
Foetal monitoring
The strong association of MAS with foetal distress has long been known1,71. Continuous foetal heart rate (FHR) monitoring has been routinely used to detect precursors of foetal asphyxia, allowing for early intervention to reduce the risk of adverse neonatal outcomes. In the context of MSAF, routine FHR monitoring has been recommended to screen for early signs of foetal hypoxia72.
Foetal pulse oximetry has shown potential as an effective method for monitoring pregnancies complicated by meconium passage. In a small study conducted by Carbonne and colleagues, foetal pulse oximeters were placed against the cheek or temple of meconium-stained infants. Throughout the progression of labour, infants who later developed MAS exhibited consistent and progressive decreases in oxygen saturation compared to infants who did not develop MAS73.
Management of infant born through MSAF
The management of infants born through MSAF has evolved over the years based on emerging evidence from clinical trials and observational studies74,75.
In the late 1960s and early 1970s, the prevailing belief was that airway obstruction by meconium was the primary cause of MAS. Consequently, during the 1970s and 1980s, the standard practice involved oro- and nasopharyngeal suctioning before the delivery of the shoulders and immediate postnatal intubation and tracheal suctioning to prevent MAS in infants born through MSAF. This approach was largely based on many single-centre, non-randomized, or prospective observational studies that demonstrated a decrease in the incidence of MAS in suctioned groups76–78. As a result, oro- and nasopharyngeal suctioning became the recommended standard of care for births complicated by thick or particulate MSAF in the first three editions of the Neonatal Resuscitation Program (NRP) textbook79.
However, in the late 1980s and early 1990s, there were conflicting results from studies investigating the efficacy of routine endotracheal suctioning for preventing MAS in vigorous MSAF neonates. In 1988, Linder et al.80 performed a single-centre RCT to evaluate the effect of intubation and suctioning on 572 vigorous neonates delivered vaginally with MSAF. In this study, endotracheal suctioning did not decrease the incidence of MAS and there were some complications (hoarseness, stridor) related to intubation.
Following the publication of a multicenter, randomized study by Wiswell and colleagues in 200081, which showed no benefit of routine intubation for vigorous MSAF neonates, the 2000 American Heart Association (AHA) guidelines82 recommended selective tracheal suctioning only for non-vigorous newborns with MSAF.
The publication of the multicenter RCT by Vain and colleagues83 in 2004 further contributed to changes in the management of infants born through MSAF. This study provided additional evidence that oro- and nasopharyngeal suctioning during the intrapartum period did not offer any significant benefits in preventing MAS.
Following the publication of this study, the 2005 AHA guidelines, the fifth edition of the NRP textbook, and the American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice69,84 no longer recommended routine intrapartum suctioning. However, endotracheal intubation and suctioning for non-vigorous neonates were still recommended due to insufficient evidence to change practice in 2005 and 201084,85.
In 2015 and 2016, two randomized studies regarding the efficacy of routine intubation for non-vigorous MSAF neonates were published15,86. Both studies did not show any significant benefit of routine endotracheal intubation and suctioning in non-vigorous neonates with MSAF. Considering the potential risks associated with delaying PPV and the lack of clear evidence supporting routine endotracheal intubation, the seventh edition of the NRP textbook published in 2016, recommended against routine endotracheal intubation for non-vigorous MSAF neonates87.
According to the updated guidelines88, if an infant born through MSAF is non-vigorous and has inadequate breathing efforts after delivery, the initial steps of resuscitation should be completed under the radiant warmer. If the infant still does not show adequate breathing efforts or has a heart rate of less than 100 beats per minute after the initial steps, PPV should be initiated. Tracheal suction with a meconium aspirator is indicated if airway obstruction prevents effective PPV.
Future therapy
The management of MAS remains primarily supportive, with no definitive therapy for meconium-induced lung injury. However, a deeper understanding of the pathological mechanisms causing meconium-induced parenchymal injury has expanded the horizon of potential future therapies. Recent data suggest that at least some cases of cell death induced by meconium occur through apoptosis, opening up the possibility of pharmacologic intervention using apoptosis blockers or other strategies. In animal models of lung injury, related studies have demonstrated that apoptosis of lung epithelial cells is mediated by the renin angiotensin system89,90. Pretreatment of rabbit pups with the angiotensin-converting enzyme inhibitor captopril before intratracheal instillation of meconium has demonstrated a reduction in lung epithelial cell apoptosis91. Similarly, the administration of N-acetyl cysteine, along with surfactant, has shown efficacy in preventing neutrophil migration, lung oedema, and oxidative damage by suppressing the formation of interleukin-8 and interleukin-1β92. The administration of the cyclooxygenase-2 (COX-2) inhibitor parecoxib in meconium-treated rabbits attenuates histopathologic damage and meconium-induced acute lung injury with significant improvement in respiratory functions93. Rescue therapy with intratracheal albumin has demonstrated improvement in lung function by binding and blocking active substances such as free fatty acids and bile acids in meconium94. A protease inhibitor cocktail has shown promise in preventing cell detachment induced by meconium, suggesting a potential role for foetal pancreatic digestive enzymes in MAS treatment95. Liquid ventilation with perfluorocarbons has been found beneficial in improving survival in animal models of MAS, offering research potential for its use in humans96. These therapies are still in the experimental stage, with evidence primarily derived from animal studies. Further human trials are needed to assess their safety and efficacy in a clinical context.
Conclusions
Despite advancements in obstetrical and neonatal care, MAS remains an important source of morbidity and mortality in term and post-term newborns. The lung injury caused by MAS is complex and can be attributed to a variety of factors, including mechanical obstruction of the airways, inactivation of surfactant, and chemical pneumonitis.
Among preventive strategies, elective induction of labour for pregnancies more than or equal to 41 weeks has been associated with a significant reduction in the incidence of MAS. Additionally, amnioinfusion has been found to reduce the risk of MAS, although this seems to be particularly beneficial in clinical settings where peripartum surveillance is limited.
Treatment strategies for MAS comprise mechanical ventilation, surfactant therapy, and supportive care. The use of steroid therapy, however, remains a subject of debate. Antibiotic therapy did not show any significant clinical benefits in neonates with MAS and no evidence of sepsis. The efficacy of antibiotics in MAS management may need reassessment through well-designed clinical trials.
Ethical approval
This article does not require ethical approval.
Consent
Written informed consent was obtained from the patient’s legal guardian for the publication of Figure 1 A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Conceptualization, G.D.; methodology, G.D.; investigation, G.D. and C.M.S.; writing—original draft preparation, G.D. and C.M.S.; writing—review and editing, S.C., F.C., P.G.; supervision, A.V. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Gianluca Dini.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Gianluca Dini, Email: gianlucadini90@gmail.com.
Sara Ceccarelli, Email: sara.ceccarelli@aospterni.it.
Federica Celi, Email: federica.celi@aospterni.it.
Carla Maria Semeraro, Email: carla.semeraro@gmail.com.
Paolo Gorello, Email: paolo.gorello@unipg.it.
Alberto Verrotti, Email: alberto.verrottidipianella@unipg.it.
References
- 1.Ahanya SN, Lakshmanan J, Morgan BL, et al. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv 2005;60:45–56; quiz 73-4. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Kumar P, Basu S. Endotracheal suctioning for prevention of meconium aspiration syndrome: a randomized controlled trial. Eur J Pediatr 2019;178:1825–1832. [DOI] [PubMed] [Google Scholar]
- 3.Rawat M, Nangia S, Chandrasekharan P, et al. Approach to infants born through meconium stained amniotic fluid: evolution based on evidence? Am J Perinatol 2018;35:815–822. [DOI] [PubMed] [Google Scholar]
- 4.Vain NE, Batton DG. Meconium “aspiration” (or respiratory distress associated with meconium-stained amniotic fluid?). Semin Fetal Neonatal Med 2017;22:214–219. [DOI] [PubMed] [Google Scholar]
- 5.Fanaroff AA. Meconium aspiration syndrome: historical aspects. J Perinatol 2008;28(suppl 3):S3–S7. [DOI] [PubMed] [Google Scholar]
- 6.Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspiration syndrome. an update. Pediatr Clin North Am 1998;45:511–529. [DOI] [PubMed] [Google Scholar]
- 7.Fischer C, Rybakowski C, Ferdynus C, et al. A population-based study of meconium aspiration syndrome in neonates born between 37 and 43 weeks of gestation. Int J Pediatr 2012;2012:321545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrea EM, Jr, Naqvi M. The influence of gestational age on the ability of the fetus to pass meconium in utero. Clinical implications. Acta Obstet Gynecol Scand. 1982;61:275-277. [DOI] [PubMed] [Google Scholar]
- 9.Usher RH, Boyd ME, McLean FH, et al. Assessment of fetal risk in postdate pregnancies. Am J Obstet Gynecol 1988;158:259–264. [DOI] [PubMed] [Google Scholar]
- 10.Dargaville PA, Copnell B. Australian and New Zealand Neonatal Network. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics 2006;117:1712–1721. [DOI] [PubMed] [Google Scholar]
- 11.Velaphi S, Vidyasagar D. Intrapartum and postdelivery management of infants born to mothers with meconium-stained amniotic fluid: evidence-based recommendations. Clin Perinatol 2006;33:29–42; v-vi. [DOI] [PubMed] [Google Scholar]
- 12.Sriram S, Wall SN, Khoshnood B, et al. Racial disparity in meconium-stained amniotic fluid and meconium aspiration syndrome in the United States, 1989-2000. Obstet Gynecol 2003;102:1262–1268. [DOI] [PubMed] [Google Scholar]
- 13.Malhotra N, Chanana C, Kumar S, et al. Comparison of perinatal outcome of growth-restricted fetuses with normal and abnormal umbilical artery Doppler waveforms. Indian J Med Sci 2006;60:311–317. [PubMed] [Google Scholar]
- 14.McIntire DD, Bloom SL, Casey BM, et al. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999;340:1234–1238. [DOI] [PubMed] [Google Scholar]
- 15.Nangia S, Sunder S, Biswas R, et al. Endotracheal suction in term non vigorous meconium stained neonates-A pilot study. Resuscitation 2016;105:79–84. [DOI] [PubMed] [Google Scholar]
- 16.Qian L, Liu C, Zhuang W, et al. Neonatal respiratory failure: a 12-month clinical epidemiologic study from 2004 to 2005 in China. Pediatrics 2008;121:e1115–e1124. [DOI] [PubMed] [Google Scholar]
- 17.Paudel P, Sunny AK, Poudel PG, et al. Meconium aspiration syndrome: incidence, associated risk factors and outcome-evidence from a multicentric study in low-resource settings in Nepal. J Paediatr Child Health 2020;56:630–635. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand SL, Fanaroff JM, Walsh MC. Meconium stained fluid: approach to the mother and the baby. Pediatr Clin North Am 2004;51:655–667; ix. [DOI] [PubMed] [Google Scholar]
- 19.Poggi SH, Ghidini A. Pathophysiology of meconium passage into the amniotic fluid. Early Hum Dev 2009;85:607–610. [DOI] [PubMed] [Google Scholar]
- 20.Vain NE, Szyld EG, Prudent LM, et al. What (not) to do at and after delivery? Prevention and management of meconium aspiration syndrome. Early Hum Dev 2009;85:621–626. [DOI] [PubMed] [Google Scholar]
- 21.Yamada T, Minakami H, Matsubara S, et al. Meconium-stained amniotic fluid exhibits chemotactic activity for polymorphonuclear leukocytes in vitro. J Reprod Immunol 2000;46:21–30. [DOI] [PubMed] [Google Scholar]
- 22.Zagariya A, Bhat R, Navale S, et al. Cytokine expression in meconium-induced lungs. Indian J Pediatr 2004;71:195–201. [DOI] [PubMed] [Google Scholar]
- 23.Kojima T, Hattori K, Fujiwara T, et al. Meconium-induced lung injury mediated by activation of alveolar macrophages. Life Sci 1994;54:1559–1562. [DOI] [PubMed] [Google Scholar]
- 24.Moses D, Holm BA, Spitale P, et al. Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol 1991;164:477–481. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Rodriguez E, Echaide M, Cruz A, et al. Meconium impairs pulmonary surfactant by a combined action of cholesterol and bile acids. Biophys J 2011;100:646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopincova J, Calkovska A. Meconium-induced inflammation and surfactant inactivation: specifics of molecular mechanisms. Pediatr Res 2016;79:514–521. [DOI] [PubMed] [Google Scholar]
- 27.Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, et al. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics 2017;139:e20161165. [DOI] [PubMed] [Google Scholar]
- 28.Papamatheakis DG, Blood AB, Kim JH, et al. Antenatal hypoxia and pulmonary vascular function and remodeling. Curr Vasc Pharmacol 2013;11:616–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haakonsen Lindenskov PH, Castellheim A, Saugstad OD, et al. Meconium aspiration syndrome: possible pathophysiological mechanisms and future potential therapies. Neonatology 2015;107:225–230. [DOI] [PubMed] [Google Scholar]
- 30.Corsini I, Parri N, Ficial B, et al. Lung ultrasound in the neonatal intensive care unit: Review of the literature and future perspectives. Pediatr Pulmonol 2020;55:1550–1562. [DOI] [PubMed] [Google Scholar]
- 31.Piastra M, Yousef N, Brat R, et al. Lung ultrasound findings in meconium aspiration syndrome. Early Hum Dev 2014;90(suppl 2):S41–S43. [DOI] [PubMed] [Google Scholar]
- 32.de Boode WP, Singh Y, Molnar Z, et al. Application of Neonatologist Performed Echocardiography in the assessment and management of persistent pulmonary hypertension of the newborn. Pediatr Res 2018;84(suppl 1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharya S, Sen S, Levy PT, et al. Comprehensive Evaluation of Right Heart Performance and Pulmonary Hemodynamics in Neonatal Pulmonary Hypertension: Evaluation of cardiopulmonary performance in neonatal pulmonary hypertension. Curr Treat Options Cardiovasc Med 2019;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P, Nangia S, Tiwari S, et al. Gastric lavage for prevention of feeding problems in neonates with meconium-stained amniotic fluid: a randomised controlled trial. Paediatr Int Child Health 2014;34:115–119. [DOI] [PubMed] [Google Scholar]
- 35.Garg J, Masand R, Tomar BS. Utility of gastric lavage in vigorous neonates delivered with meconium stained liquor: a randomized controlled trial. Int J Pediatr 2014;2014:204807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta R. Gastric lavage in vigorous neonates born with meconium stained amniotic fluid. Indian J Pediatr 2013;80:252. [DOI] [PubMed] [Google Scholar]
- 37.Ameta G, Upadhyay A, Gothwal S, et al. Role of gastric lavage in vigorous neonates born with meconium stained amniotic fluid. Indian J Pediatr 2013;80:195–198. [DOI] [PubMed] [Google Scholar]
- 38.Deshmukh M, Balasubramanian H, Rao S, et al. Effect of gastric lavage on feeding in neonates born through meconium-stained liquor: a systematic review. Arch Dis Child Fetal Neonatal Ed 2015;100:F394–F399. [DOI] [PubMed] [Google Scholar]
- 39.Pandita A, Murki S, Oleti TP, et al. Effect of nasal continuous positive airway pressure on infants with meconium aspiration syndrome: a randomized clinical trial. JAMA Pediatr 2018;172:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldsmith JP. Continuous positive airway pressure and conventional mechanical ventilation in the treatment of meconium aspiration syndrome. J Perinatol 2008;28(suppl 3):S49–S55. [DOI] [PubMed] [Google Scholar]
- 41.Dargaville PA. Respiratory support in meconium aspiration syndrome: a practical guide. Int J Pediatr 2012;2012:965159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canadian Pediatric Society . Recommendation for neonatal surfactant therapy. Paediatr Child Health 2004;2:109–116. [PMC free article] [PubMed] [Google Scholar]
- 43.Dargaville PA, South M, McDougall PN. Surfactant and surfactant inhibitors in meconium aspiration syndrome. J Pediatr 2001;138:113–115. [DOI] [PubMed] [Google Scholar]
- 44.El Shahed AI, Dargaville PA, Ohlsson A, et al. Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst Rev 2014;2014:CD002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly LE, Shivananda S, Murthy P, et al. Antibiotics for neonates born through meconium-stained amniotic fluid. Cochrane Database Syst Rev 2017;6:CD006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu S, Kumar A, Bhatia BD. Role of antibiotics in meconium aspiration syndrome. Ann Trop Paediatr 2007;27:107–113. [DOI] [PubMed] [Google Scholar]
- 47.Shankar V, Paul VK, Deorari AK, et al. Do neonates with meconium aspiration syndrome require antibiotics? Indian J Pediatr 1995;62:327–331. [DOI] [PubMed] [Google Scholar]
- 48.Yeung T, Jasani B, Shah PS. Steroids for the management of neonates with meconium aspiration syndrome: a systematic review and meta-analysis. Indian Pediatr 2021;58:370–376. [PubMed] [Google Scholar]
- 49.Mokra D, Mokry J, Drgova A, et al. Intratracheally administered corticosteroids improve lung function in meconium-instilled rabbits. J Physiol Pharmacol 2007;58(suppl 5 Pt 1):389–398. [PubMed] [Google Scholar]
- 50.Mikolka P, Mokrá D, Kopincová J, et al. Budesonide added to modified porcine surfactant Curosurf may additionally improve the lung functions in meconium aspiration syndrome. Physiol Res 2013;62(suppl 1):S191–S200. [DOI] [PubMed] [Google Scholar]
- 51.Garg N, Choudhary M, Sharma D, et al. The role of early inhaled budesonide therapy in meconium aspiration in term newborns: a randomized control study. J Matern Fetal Neonatal Med 2016;29:36–40. [DOI] [PubMed] [Google Scholar]
- 52.Tripathi S, Saili A. The effect of steroids on the clinical course and outcome of neonates with meconium aspiration syndrome. J Trop Pediatr 2007;53:8–12. [DOI] [PubMed] [Google Scholar]
- 53.Walsh MC, Fanaroff JM. Meconium stained fluid: approach to the mother and the baby. Clin Perinatol 2007;34:653–665; viii. [DOI] [PubMed] [Google Scholar]
- 54.Wessel DL, Adatia I, Van Marter LJ, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1997;100:E7. [DOI] [PubMed] [Google Scholar]
- 55.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2006:CD000399. doi: 10.1002/14651858.CD000399.pub2Update in: Cochrane Database Syst Rev. 2017 Jan 05;1:CD000399. [DOI] [PubMed] [Google Scholar]
- 56.Konduri GG, Solimano A, Sokol GM, et al. Neonatal Inhaled Nitric Oxide Study Group . A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics 2004;113(3 Pt 1):559–564. [DOI] [PubMed] [Google Scholar]
- 57.Asad A, Bhat R. Pharmacotherapy for meconium aspiration. J Perinatol 2008;28(suppl 3):S72–S78. [DOI] [PubMed] [Google Scholar]
- 58.Short BL. Neonatal ECMO: are indications changing? Int J Artif Organs 1995;18:562–564. [PubMed] [Google Scholar]
- 59.Kanto WP, Jr. A decade of experience with neonatal extracorporeal membrane oxygenation. J Pediatr 1994;124:335–347. [DOI] [PubMed] [Google Scholar]
- 60.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;2013:CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyckoff MH Wyllie J Aziz K et al. Neonatal Life Support Collaborators . Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2020;142(16_suppl_1):S185–S221. [DOI] [PubMed] [Google Scholar]
- 62.Singh BS, Clark RH, Powers RJ, et al. Meconium aspiration syndrome remains a significant problem in the NICU: outcomes and treatment patterns in term neonates admitted for intensive care during a ten-year period. J Perinatol 2009;29:497–503. [DOI] [PubMed] [Google Scholar]
- 63.Wiswell TE, Tuggle JM, Turner BS. Meconium aspiration syndrome: have we made a difference? Pediatrics 1990;85:715–721. [PubMed] [Google Scholar]
- 64.Gülmezoglu AM, Crowther CA, Middleton P, et al. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2012;6:CD004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vázquez Nava F, Salas Ramírez E, Sánchez Núncio HR, et al. Meconium aspiration syndrome, parental atopy and asthma symptoms in children under two years old. Rev Alerg Mex 2006;53:130–135. [PubMed] [Google Scholar]
- 66.Beligere N, Rao R. Neurodevelopmental outcome of infants with meconium aspiration syndrome: report of a study and literature review. J Perinatol 2008;28(suppl 3):S93–S101. [DOI] [PubMed] [Google Scholar]
- 67.Pierce J, Gaudier FL, Sanchez-Ramos L. Intrapartum amnioinfusion for meconium-stained fluid: meta-analysis of prospective clinical trials. Obstet Gynecol 2000;95(6 Pt 2):1051–1056. [DOI] [PubMed] [Google Scholar]
- 68.Hofmeyr GJ, Xu H, Eke AC. Amnioinfusion for meconium-stained liquor in labour. Cochrane Database Syst Rev 2014;2014:CD000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.ACOG Committee Obstetric Practice . ACOG Committee Opinion Number 346, October 2006: amnioninfusion does not prevent meconium aspiration syndrome. Obstet Gynecol 2006;108:1053. [DOI] [PubMed] [Google Scholar]
- 70.Hussain AA, Yakoob MY, Imdad A, et al. Elective induction for pregnancies at or beyond 41 weeks of gestation and its impact on stillbirths: a systematic review with meta-analysis. BMC Public Health 2011;11(Suppl 3):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown CA, Desmond MM, Lindley JE, et al. Meconium staining of the amniotic fluid; a marker of fetal hypoxia. Obstet Gynecol 1957;9:91–103. [PubMed] [Google Scholar]
- 72.MacDonald D, Grant A, Sheridan-Pereira M, et al. The Dublin randomized controlled trial of intrapartum fetal heart rate monitoring. Am J Obstet Gynecol 1985;152:524–539. [DOI] [PubMed] [Google Scholar]
- 73.Carbonne B, Cudeville C, Sivan H, et al. Fetal oxygen saturation measured by pulse oximetry during labour with clear or meconium-stained amniotic fluid. Eur J Obstet Gynecol Reprod Biol 1997;72(suppl):S51–S55. [DOI] [PubMed] [Google Scholar]
- 74.Gandhi CK. Management of meconium-stained newborns in the delivery room. Neonatal Netw 2018;37:141–148. [DOI] [PubMed] [Google Scholar]
- 75.Chabra S. Evolution of delivery room management for meconium-stained infants: recent updates. Adv Neonatal Care 2018;18:267–275. [DOI] [PubMed] [Google Scholar]
- 76.Gregory GA, Gooding CA, Phibbs RH, et al. Meconium aspiration in infants—a prospective study. J Pediatr 1974;85:848–852. [DOI] [PubMed] [Google Scholar]
- 77.Ting P, Brady JP. Tracheal suction in meconium aspiration. Am J Obstet Gynecol 1975;122:767–771. [DOI] [PubMed] [Google Scholar]
- 78.Carson BS, Losey RW, Bowes WA, Jr, et al. Combined obstetric and pediatric approach to prevent meconium aspiration syndrome. Am J Obstet Gynecol 1976;126:712–715. [DOI] [PubMed] [Google Scholar]
- 79.Burchfield D, Erenberg A, Mullett MD, et al. Why change the compression and ventilation rates during CPR in neonates? Neonatal Resuscitation Steering Committee, American Heart Association and American Academy of Pediatrics. Pediatrics 1994;93(6 Pt 1):1026–1027. [PubMed] [Google Scholar]
- 80.Linder N, Aranda JV, Tsur M, et al. Need for endotracheal intubation and suction in meconium-stained neonates. J Pediatr 1988;112:613–615. [DOI] [PubMed] [Google Scholar]
- 81.Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics 2000;105(1 Pt 1):1–7. [DOI] [PubMed] [Google Scholar]
- 82.Niermeyer S, Kattwinkel J, Van Reempts P, et al. International Guidelines for Neonatal Resuscitation: An excerpt from the Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International Consensus on Science. Contributors and Reviewers for the Neonatal Resuscitation Guidelines. Pediatrics 2000;106:E29. [DOI] [PubMed] [Google Scholar]
- 83.Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet 2004;364:597–602. [DOI] [PubMed] [Google Scholar]
- 84.American Heart Association . 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics 2006;117:e989–e1004. [DOI] [PubMed] [Google Scholar]
- 85.Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal Resuscitation Chapter Collaborators. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2010;122(16 suppl 2):S516–S538. [DOI] [PubMed] [Google Scholar]
- 86.Chettri S, Adhisivam B, Bhat BV. Endotracheal suction for nonvigorous neonates born through meconium stained amniotic fluid: a randomized controlled trial. J Pediatr 2015;166:1208–1213.e1. [DOI] [PubMed] [Google Scholar]
- 87.Weiner GM. Textbook of neonatal resuscitation. American Academy; 2016. [Google Scholar]
- 88.Aziz K, Lee HC, Escobedo MB, et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020;142(16_suppl_2):S524–S550. [DOI] [PubMed] [Google Scholar]
- 89.Li X, Shu R, Filippatos G, et al. Apoptosis in lung injury and remodeling. J Appl Physiol (1985) 2004;97:1535–1542. [DOI] [PubMed] [Google Scholar]
- 90.Wang R, Zagariya A, Ibarra-Sunga O, et al. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 1999;276:L885–L889. [DOI] [PubMed] [Google Scholar]
- 91.Uhal BD, Abdul-Hafez A. Angiotensin II in apoptotic lung injury: potential role in meconium aspiration syndrome. J Perinatol 2008;28(suppl 3):S108–S112. [DOI] [PubMed] [Google Scholar]
- 92.Kopincová J, Mokrá D, Mikolka P, et al. N-acetylcysteine advancement of surfactant therapy in experimental meconium aspiration syndrome: possible mechanisms. Physiol Res 2014;63(suppl 4):S629–S642. [DOI] [PubMed] [Google Scholar]
- 93.Li AM, Zhang LN, Li WZ. Amelioration of meconium-induced acute lung injury by parecoxib in a rabbit model. Int J Clin Exp Med 2015;8:6804–6812. [PMC free article] [PubMed] [Google Scholar]
- 94.Saugstad OD, Tølløfsrud PA, Lindenskov P, et al. Toxic effects of different meconium fractions on lung function: new therapeutic strategies for meconium aspiration syndrome? J Perinatol 2008;28(suppl 3):S113–S115. [DOI] [PubMed] [Google Scholar]
- 95.Ivanov VA, Gewolb IH, Uhal BD. A new look at the pathogenesis of the meconium aspiration syndrome: a role for fetal pancreatic proteolytic enzymes in epithelial cell detachment. Pediatr Res 2010;68:221–224. [DOI] [PubMed] [Google Scholar]
- 96.Jeng MJ, Soong WJ, Lee YS, et al. Effects of therapeutic bronchoalveolar lavage and partial liquid ventilation on meconium-aspirated newborn piglets. Crit Care Med 2006;34:1099–1105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.