Abstract
Introduction and importance:
Systemic lupus erythematosus (SLE) predominantly affects young women and is associated with an increased risk of thrombosis. Antiphospholipid antibody syndrome (APS) may complicate the clinical picture, often leading to recurrent arteriovenous thrombosis. This case report underscores the significance of two unique aspects: the rare occurrence of an atrial thrombus and the presence of antinuclear antibody (ANA)-negative SLE.
Case presentation:
A 32-year-old woman presented with a history of symmetric polyarticular joint pain, oral ulcers, significant weight loss, and a history of unprovoked popliteal thrombosis and two unexplained abortions. One week prior to admission, she experienced severe headaches and elevated blood pressure. Clinical evaluation revealed several abnormalities, including a systolic murmur, livedo reticularis, and a transthoracic echocardiogram showing severe mitral regurgitation and an atrial thrombus. A transesophageal echocardiogram confirmed the presence of a pedunculated lesion in the right atria, challenging differential diagnosis.
Clinical discussion:
ANA-negative SLE, though rare, was observed in this patient, highlighting diagnostic complexities. APS compounded the clinical presentation, emphasizing the importance of identifying specific autoantibodies and recurrent thrombotic events. In the case of atrial thrombus, differentiation from other cardiac conditions, such as myxoma or vegetation, is a key.
Conclusions:
This case underscores the critical importance of recognizing and managing atrial thrombus, a rare but life-threatening complication in patients with systemic lupus erythematosus and antiphospholipid syndrome. Additionally, the diagnostic challenge of ANA-negative SLE warrants careful consideration in patients presenting with characteristic features of the disease.
Keywords: antiphospholipid syndrome, atrial thrombus, autoimmune disorders, systemic lupus erythematosus, thrombosis
Introduction
Highlights
Systemic lupus erythematosus (SLE) predominantly affects young women and is associated with an increased risk of thrombosis.
Antiphospholipid antibody syndrome (APS) can complicate the clinical picture of SLE, leading to recurrent arteriovenous thrombosis.
This case report highlights the rare occurrence of an atrial thrombus, a life-threatening complication in SLE and APS.
The presence of antinuclear antibody (ANA)-negative SLE in this patient adds to the diagnostic complexities of the disease.
Differentiating atrial thrombus from other cardiac conditions such as myxoma is crucial in diagnosis.
ANA-negative lupus, although rare, challenges conventional diagnostic criteria for SLE.
Systemic lupus erythematosus (SLE) is an autoimmune disease with diverse clinical manifestations that primarily affects young women. Women are affected nine times more frequently than men1. Patients with SLE have an increased risk for thrombosis. Arterial and/or venous thrombosis is a well-known clinical entity in SLE, with a prevalence of more than 10%. This prevalence may even exceed 50% in high-risk patients2 The age at onset of thrombosis in SLE patients is lower than that of the general population which is a major concern. Antiphospholipid antibody syndrome (APS) is an autoimmune disease associated with the presence of antiphospholipid antibodies such as lupus anticoagulant (LAC), anti-cardiolipin antibodies, or anti-β2 glycoprotein-I antibodies, thus resulting in recurrent arteriovenous thrombosis and recurrent foetal loss or failure to thrive. When left untreated, arterial and venous thrombosis has been reported to recur in 50% of cases within 6 months of pregnancy3.
The following case of a 32-year-old woman who presented with signs and symptoms of SLE and APS is of significant importance due to two distinct and noteworthy aspects: the rare occurrence of an atrial thrombus and the presence of antinuclear antibody (ANA)-negative SLE.
By presenting this case, we highlight the uncommon manifestation of an atrial thrombus in a patient with SLE and APS, shedding light on the need for a high index of suspicion and timely diagnosis to prevent potentially fatal consequences.
This case has been reported in line with the SCARE 2023 criteria4.
Case presentation
A 32-year-old woman presented to the rheumatology department with a 5-year history of symmetric polyarticular joint pain in hands, elbows, and knees responsive to NSAIDs, with recurrent painless oral ulcers. During this period, she also had a significant loss of appetite with weight loss of 10 kg. The patient has a previous history of unprovoked popliteal thrombosis in the lower extremity, and remarkably, there are no identified risk factors for venous thromboembolism (VTE). There is no history of cardiac failure, respiratory failure, recent stroke, recent myocardial infarction, acute infectious disease, malignancy, myeloproliferative syndrome, nephrotic syndrome, previous VTE, prolonged immobilization, travel exceeding 6 h, or chronic venous insufficiency. Furthermore, the patient has not undergone any hormonal therapy, be it contraceptive or substitutive.
She had two unexplained abortions at 24 weeks of gestation, she also mentioned having elevated blood pressure during pregnancy (Preeclampsia without severe features), she was treated with aspirin and heparin, and thereafter she had full-term delivery.
One week prior to admission the patient complained of severe headache with elevated blood pressure of 200/110 mmhg. She didn’t have any medication at that time.
On evaluation, the BMI was 16.6 kg /m2. Temperature was 36.4°C, blood pressure was 140/90 mmHg, pulse 88 beats/min, respiration rate 20 /min, and oxygen saturation of 98% in room air. There were no lower limb oedema and no crackles was found on lung auscultation. Cardiovascular examination revealed a grade III systolic murmur in the mitral area radiating to the axilla with soft S1 and normal S2. Livedo reticularis was evident on the feet. The rest of the examination was unremarkable.
A transthoracic echocardiogram (TTE) was conducted and showed severe mitral regurgitation, left ventricular hypokinesia, and thrombus in patent foramen ovale. Systolic pulmonary artery pressure was estimated to be 35 mmHg.
Subsequently, a transesophageal echocardiogram (TEE) revealed an absence of clots in the left atrium and appendage with a good emptying velocity.
A bubble test was performed and demonstrated a protracted diaphragm separating the two atria, along with a lesion measuring 1.5 × 1.56 cm2. The lesion was hyperechoic, and mobile, with calcification at its apex in proximity to the inferior vena cava, suggesting a potential diagnosis of a haemorrhagic thrombus or chronic vegetation (Fig. 1).
Figure 1.
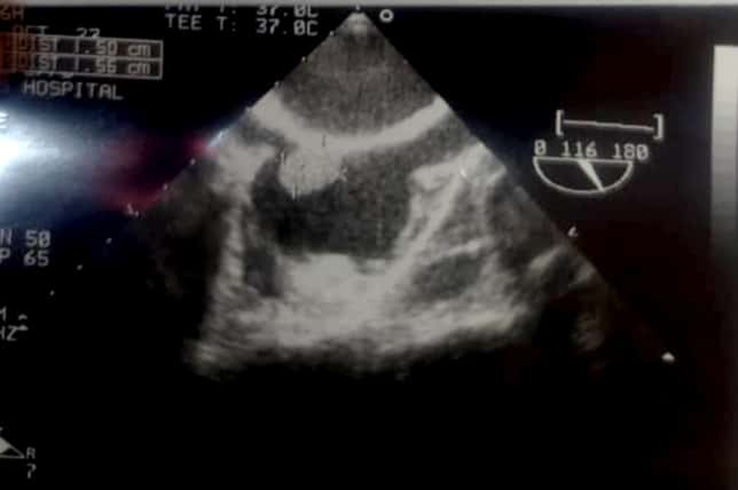
An ultrasound of the right atria demonstrating a 1.5×1.56 cm2 hyperechoic pedunculated lesion.
Laboratory investigations revealed: anaemia, indicated by a haemoglobin level of 8.8 g/dl; a mean corpuscular volume (MCV) of 82 fl; and thrombocytopenia, with a platelet count of 98 ×103/µl. The glomerular filtration rate (GFR) was 110.
Mixing studies, which are tests conducted on the blood plasma of patients or test subjects, play a key role in differentiating coagulation factor deficiencies from factor inhibitors. Examples of inhibitors include lupus anticoagulant or specific factor inhibitors like antibodies directed against factor VIII. Specifically in our case, the mixing study results were as follows: before mixing, the prothrombin time (PT) was 53, PTT was 35, and the international normalized ratio (INR) was 1. After mixing, the PT remained at 53, the PTT slightly decreased to 33, and the INR remained unchanged at 1. Blood cultures were negative. (Table 1)
Table 1.
Routine laboratory tests
| Test | Result | Normal range |
|---|---|---|
| White blood cell (WBC) | 6.7 | 4.4–11 ×103/µl |
| Lymphocyte | 31 | 20–40% |
| Neutrophil | 62 | 40–70% |
| Haemoglobin (Hb) | 8.8 | 12–14 g/dl |
| Mean corpuscular volume (MCV) | 82 | 80–96 fl |
| Haematocrit (Hct) | 25.1 | 37–42% |
| Platelet count | 98 | 150–450 ×103/µl |
| Red cell distribution width (RDW) | 15.4 | 11.5–14.5% |
| Erythrocyte sedimentation rate (ESR) | 80 | Up to 0.5 mm/h |
| C-reactive protein (CRP) | 1.46 | Up to 0.5 mg/dl |
| Urea (Ur) | 85 | 10–50 mg/dl |
| Creatinine (Cr) | 1.52 | 0.6–1.2 mg/dl |
| Glucose (Glu) | 88 | 70–110 mg/dl |
| Lactate dehydrogenase (LDH) | 300 | Up to 250 U/l |
| Albumin | 4.5 | 3.4–4.5 g/dl |
| Sodium (Na) | 139 | 135–140 mEq/l |
| Potassium (K) | 4 | 3.5–5 mEq/l |
| Chloride (Cl) | 101 | 95–105 mEq/l |
| Prothrombin time (PT) | 63 | 85–100% |
| International normalized ratio (INR) | 1.37 | 0.8–0.1 |
| Partial thromboplastin time (PTT) | 53 sec | 25–35 sec |
| Reticulocyte | 0.97 | 0.5–1.5% |
| Aerobic blood culture | Negative | — |
The level of anti-cardiolipin (IgG) was 27.3 (normal <15) and anti-beta-2 -glycoprotein (IgG) was 121 (normal <20), while tests for ANA and anti-double-stranded antinuclear antibodies (dsdNA) yielded negative results. Additionally, complement proteins C3 and C4 were found to be low. Rheumatoid factor (Rf) was negative, and anti-cyclic citrullinated peptide (Anti-CCP) testing was not conducted. (Table 2).
Table 2.
Immunological tests
| Test | Result | Normal range |
|---|---|---|
| Antinuclear antibody (ANA) | Negative | |
| Anti-double-stranded DNA antibody (anti-DsDNA Ab) | Negative | |
| Complement C3 | 56 | 70–196 mg/dl |
| Complement C4 | 5 | 12–40 mg/dl |
| Direct Coombs test | Negative | |
| Indirect Coombs test | Negative | |
| Anti-cardiolipin IgG | 27.3 | Up to 10 GPL Units |
| Anti-cardiolipin IgM | 8.54 | Up to 10 MPL Units |
| Anti-b2 glycoprotein IgG | 121 | Up to 18 GPL Units |
| Anti-b2 glycoprotein IgM | 9.6 | Up to 18 MPL Units |
| Lupus anticoagulant | Negative | |
| Anti-SSA (RO) antibody | 5.14 | Up to 10 Units |
| Anti-SSB (LA) antibody | 6.88 | Up to 10 Units |
Urinalysis showed moderate proteinuria (Table 3).
Table 3.
Urinalysis
| Test | Result | Normal range |
|---|---|---|
| Total protein to creatinine ratio (morning urine) | 0.48 | Up to 0.2 |
| Creatinine (Cr) in morning urine | 48.8 | 20–275 mg/dl |
| Protein | ++ | |
| Casts | — | — |
| Red blood cells (RBC) | 5 | 0–5 per high-power field (HPF) |
| White blood cells (WBC) | 5 | 0–5 per high-power field (HPF) |
| Colour | Yellow | Yellow |
| Specific gravity | 1.010 | 1.005–1.030 |
| pH | 5 | 4.6–8.0 |
The patient fulfilled the criteria of SLE with APS which will be clearly explained in the following discussion.
TEE was done after 6 weeks for follow-up, and the patient symptoms significantly improved.
Discussion
SLE is a chronic autoimmune disorder characterized by immune dysregulation and inflammation that can affect multiple organ systems5. The development of SLE involves several immunopathogenic pathways6.
The diagnosis of SLE is determined by the presence of various clinical and laboratory criteria. These include skin rashes, mucosal ulcers, arthritis, serositis, renal involvement, neurological manifestations, blood abnormalities (such as leukopenia and thrombocytopenia), with the existence of specific autoantibodies, such as ANA and dsDNA antibodies. According to EULAR/ACR 2019 SLE classification criteria, ANAs are an obligatory entry criterion7.
However, our patient results demonstrated a negative ANA test despite other characteristic features of SLE diagnosis.
Those ANA-negative SLE patients would be missed if based on the 2019 (EULAR)/ACR classification criteria for SLE, which also affected the sensitivity of this classification criteria8.
Therefore, for a suspected ANA-negative SLE, the diagnosis should be established according to the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria. The SLICC criteria require either that a patient satisfy at least 4 of 17 criteria, including at least 1 of the 11 clinical criteria and one of the six immunologic criteria, OR that the patient has biopsy-proven nephritis compatible with SLE in the presence of ANA or dsDNA antibodies. The clinical criteria are related to the manifestations of SLE in different organs and systems, such as skin, joints, kidneys, blood cells, and the nervous system. The immunologic criteria are related to the presence of autoantibodies that indicate an autoimmune process, such as ANA, anti-dsDNA, anti-Sm, antiphospholipid antibody, low complement level, and direct Coombs test9.
The patient met clinical criteria (3 out of 11) with manifestations such as oral ulcers, arthritis, and thrombocytopenia. In terms of immunologic criteria, the patient test results reviled positive antiphospholipid antibodies and low complement levels (2 out of 6).
The concept of ANA-negative lupus was first introduced by Koller and colleagues in 1976. The authors reported five patients who displayed clinical features consistent with SLE despite a negative ANA test result. However, the current and latest evidence from the literature and experience in large centres suggests that true ANA-negative lupus is an extremely rare event10,11.
The prevalence of ANA-negative SLE is very low, but it exists, particularly under the influence of prolonged use of glucocorticoids or immunosuppressants or those with sever proteinuria. Low complement, thrombocytopenia, positive anti-dsDNA, and medium-high titre antiphospholipid antibody (aPL) are the main manifestations of ANA-negative SLE. So, we should identify complement, anti-dsDNA, and aPL in ANA-negative patients with rheumatic symptoms, particularly thrombocytopenia12.
APS, also known as anti-cardiolipin syndrome, is an autoimmune disorder that could occur alone (primary APS) or with other connective tissue disorders (secondary APS) and it is commonly associated with SLE. APS is characterized by the presence of specific autoantibodies, namely lupus anticoagulant (LA), anti-cardiolipin antibodies (aCL), and anti-beta-2 glycoprotein-I (anti-β2GPI) antibodies, along with a predisposition to arterial and venous thrombosis.
aPL antibodies can activate platelets, endothelial cells and monocytes. Endothelial cell activation leads to the release of proinflammatory cytokines and increased leucocyte adhesion, making possible the activation of the polymorphonuclear leucocyte. Monocyte activation leads to the release of proinflammatory cytokines as well including tissue factor, which can potentiate coagulation factor activation and ultimately fibrin production. Platelet activation leads to the release of thromboxane-B2, which potentiates the increased expression of GPIIb/IIIa, a major fibrinogen receptor. The net effect is the induction of a procoagulant state ultimately leading to thrombosis13.
aPL antibodies are found in ~30–40% of patients with SLE, but only about 10% have APS14.
The diagnosis of APS requires the presence of one or more antiphospholipid antibodies such as LA, aCL, or anti-β2GPI in moderate or high titres that remain persistently elevated on at least two separate occasions, with a minimum interval of 12 weeks between the tests. Additionally, there must be at least one event of vascular thrombosis or recurrent pregnancy loss15,16,17.
However, recently the new 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria included an entry criterion of at least one positive aPL test within 3 years of identification of an aPL-associated clinical criterion, followed by additive weighted criteria clustered into six clinical domains and two laboratory domains. The diagnosis of APS can be established according to the new criteria if the patient met the entry criterion and scored at least 6 points based on the weighted criteria from both clinical (3 points) and laboratory (3 points) domains18.
According to the 2023 criteria for APS, our patient has had the following clinical criteria:
Venous thrombosis without high-risk profile (3 points), thrombocytopenia: (2 points) preeclampsia before 34 weeks (3 points), along with the following laboratory criteria: Positive lupus anticoagulant (Single—one time) (1 point), and highly positive anti-b2 glycoprotein IgG which was 121 (normal range < 20 units) (5 points).
Thrombotic events such as stroke, transient ischaemic attack, deep vein thrombosis, and pulmonary embolism are commonly observed in patients with APS.
Although rare, intracardiac thrombus is a life-threatening complication that can increase morbidity and mortality in patients with APS19.
An atrial thrombus may appear as an intracavitary mass on echocardiography. It should be differentiated from vegetation and tumours. The most common primary cardiac tumour is myxoma which could be challenging to distinguish from a large atrial thrombus15. Mobility with cardiac activity and the absence of any additional masses in the left atrial appendage favour the diagnosis of cardiac myxoma.
Vegetations due to endocarditis are differentiated by the clinical signs and symptoms and the positive cultures.
A literature review by Dhibar et al. 16. indicated that primary APS was more common than secondary APS in 28 published cases of APS with intracardiac thrombus. Only a few cases of secondary APS were found, with SLE being the most frequent association (As in our case). Furthermore, they suggested that the right atrium was most frequently involved followed by the right ventricle.
Our case of a 32-year-old woman with ANA-negative SLE complicated with secondary APS and right atrial thrombus presents a unique clinical scenario that can be life-threatening. Notably, this case demonstrated a rare occurrence of ANA-negative lupus, challenging conventional diagnostic criteria. Early diagnosis and management of these patients are crucial to prevent life-threatening complications.
Conclusions
Negativity ANA should not exclude the diagnosis of SLE and its complications when the clinical presentation is completable with the diagnosis. The diagnostic process may further be complicated by the coexistence of APS. In this case, despite the absence of ANA, the clinical and laboratory criteria for both SLE and APS were met. This highlights the need for a comprehensive evaluation that considers various diagnostic criteria.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
There was no funding for this paper.
Author contribution
S.H. was involved in the patient care and management, data collection and wrote the main draft of the manuscript. Z.M. participated in patient care, preparing and writing of the manuscript. M.A. participated in writing the final manuscript, prepared figures, tables, revised and submitted the manuscript to the journal. S.A. supervised the case. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare no conflict of interests.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Suaad Hamsho.
Data availability statement
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Suaad Hamsho, Email: soha.hamsho3@gmail.com.
Mohammed Alaswad, Email: dr.aswad97@gmail.com.
Zeina Makhlouf, Email: zmakhlouf20@gmail.com.
Salwa Alcheikh, Email: salwacheikh@gmail.com.
References
- 1.Tassiulas IO, Boumpas DT. Clinical features and treatment of systemic lupus erythematosus. Textbook of Rheumatology. Saunders/Elsevier; 8th edn. 2009. G S Firestein and W N Kelley, eds. [Google Scholar]
- 2.Afeltra A, Vadacca M, Conti L, et al. Thrombosis in systemic lupus erythematosus: congenital and acquired risk factors. Arthritis Rheum 2005;53:452–459. [DOI] [PubMed] [Google Scholar]
- 3.Cervera R. Antiphospholipid syndrome. Thromb Res 2017;151:S43–S47. [DOI] [PubMed] [Google Scholar]
- 4.Sohrabi C, Mathew G, Maria N, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2023;109:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan HB, Li YM. Atrial thrombus as a complication of SLE and APS in an 8-year-old child. Pediatr Rheumatol 2020;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justiz Vaillant AA, Goyal A, Varacallo M. Systemic Lupus Erythematosus. 2023 Aug 4. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 30571026. [PubMed]
- 7.Aringer M, Johnson SR. Classifying and diagnosing systemic lupus erythematosus in the 21st century. Rheumatology (United Kingdom) 2020;59:V4–V11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019;71:1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koller SR, Johnston CL, Moncure CW, et al. Lupus erythematosus cell preparation-antinuclear factor incongruity: a review of diagnostic tests for systemic lupus erythematosus. Am J Clin Pathol 1976;66:495–505. [DOI] [PubMed] [Google Scholar]
- 11.Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity—does it no longer exist? QJM Int J Med 2004;97:303–308. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Zheng Y, Chen L, et al. Antinuclear antibody-negative systemic lupus erythematosus: How many patients and how to identify? Arch Rheumatol 2022;37:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper BE, Wills R, Pierangeli SS. Pathophysiological mechanisms in antiphospholipid syndrome. Int J Clin Rheumtol 2011;6:157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockshin MD. Update on antiphospholipid syndrome. Bull NYU Hosp Jt Dis 2008;66:195–197. [PubMed] [Google Scholar]
- 15.Cianciulli TF, Saccheri MC, Redruello HJ, et al. Right atrial thrombus mimicking myxoma with pulmonary embolism in a patient with systemic lupus erythematosus and secondary antiphospholipid syndrome. Tex Heart Inst J 2008;35:454–457. [PMC free article] [PubMed] [Google Scholar]
- 16.Dhibar DP, Sahu KK, Varma SC, et al. Intra-cardiac thrombus in antiphospholipid antibody syndrome: An unusual cause of fever of unknown origin with review of literature. J Cardiol Cases 2016;14:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bustamante JG, Goyal A, Singhal M. Antiphospholipid Syndrome. 2023 Feb 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 28613698. [PubMed]
- 18.Barbhaiya M, Zuily S, Naden R, et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol 2023;75:1687–1702. [DOI] [PubMed] [Google Scholar]
- 19.Abdelmahmuod EA, Hamad A, Almaghraby A, et al. Development of intracardiac thrombus in a young patient with antiphospholipid syndrome while she was on rivaroxaban: Case report and literature review. Clin Case Rep 2021;9:e04137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


