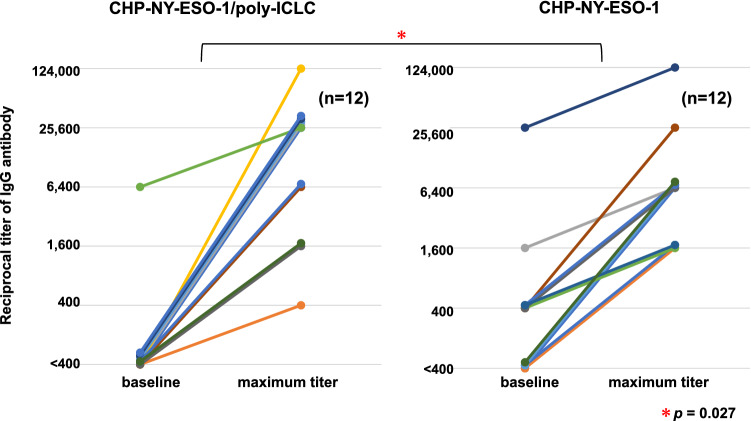
Fig. 2.
Titers of serum anti-NY-ESO-1 antibody at baseline and maximum titers after CHP-NY-ESO-1 vaccinations. Six esophageal cancer patients were enrolled in cohort 1. They were all vaccinated with 6 cycles of vaccination, and were evaluated for immune responses. In cohort 2, 10 patients were enrolled, in whom six were given six cycles of vaccination. They were evaluated for immune responses. In total, 12 patients were evaluated for immune responses. The left panel displays the changes in the 12 patients vaccinated with CHP-NY-ESO-1 (200 μg) and poly-ICLC (0.5 mg or 1.0 mg). The right panel displays the changes in 12 esophageal cancer patients vaccinated with CHP-NY-ESO-1 (200 μg) alone. The amplification of antibody titers was significantly higher in the CHP-NY-ESO1/poly-ICLC group than those in the CHP-NY-ESO-1 alone group (p = 0.027). Changes in the multiplier number (400 × 4n) of serum dilutions were statistically tested (Student t test)