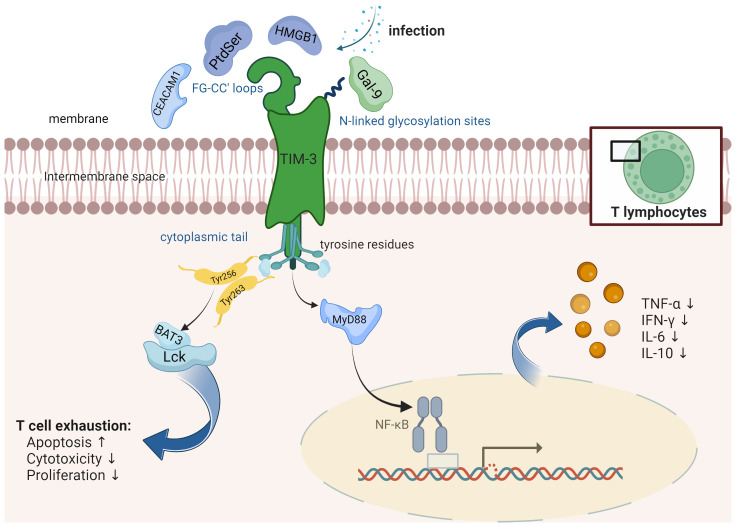
Figure 1.
TIM-3 is a protein that consists of several distinct structural elements, namely, an extracellular immunoglobulin variable (IgV) domain, a mucin stalk featuring N- and O-linked glycosylation sites, and an intracellular tail containing conserved tyrosine residues. It is found to be expressed on various immune cells including T cells, natural killer (NK) cells, and antigen-presenting cells (APCs). TIM-3 interacts with different receptors such as Phosphatidylserine (PtdSer), carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and high-mobility group box 1 (HMGB1). These receptors bind to specific regions known as the FG-CC’ loops on TIM-3. Additionally, Galectin-9 (Gal-9), a distinct protein, binds specifically to the N-linked glycosylation sites on TIM-3. TIM-3 plays a significant role in modulating the NF-κB pathway in the context of infections, thereby exerting control over the production and release of cytokines. Human Leukocyte Antigen-B-associated transcript 3 (BAT3) is an essential adaptor protein known for its interactions with the cytoplasmic domain of TIM-3. BAT3 plays a critical role in the regulation of T cell exhaustion, a state characterized by impaired effector function and increased expression of inhibitory receptors.