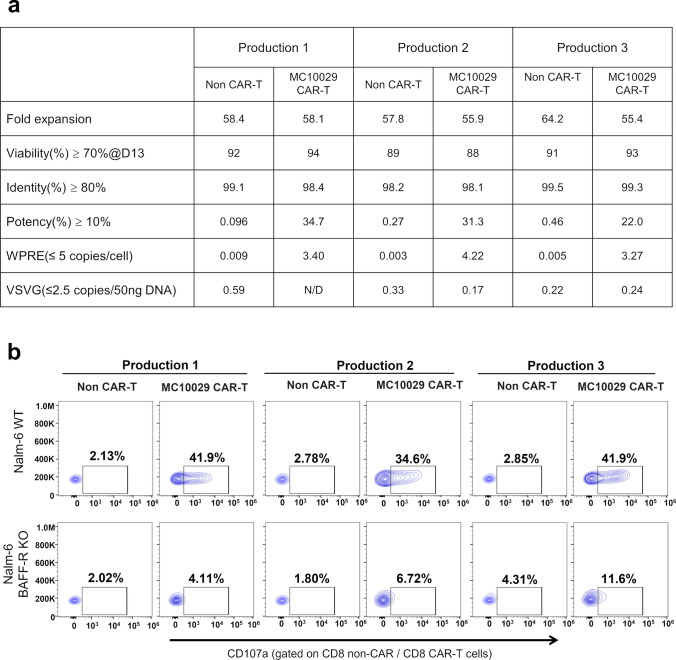
Fig. 6.
Characterization of three qualification productions of clinical-grade MC10029 CAR-T cells. a Product release criteria for CAR-T cells. Three batches of MC10029 CAR-T cells were evaluated for cell quality with fold expansion (> 25) and viability (> 70%, as determined by Trypan Blue staining) as well as CAR-T cell specific characterization with identity (> 80%, as determined by flow cytometry for CD3 positive cells) and potency (> 10%, as determined by flow cytometry for EGFR (a transgene) positive T cells). The corresponding non-CAR-T cells were used as controls. To show that the lentiviral vector remains non-infectious and safe from adventitious viral agents, the lentiviral copy number (< 5 copies/per cell of WPRE) and VSVG (< 5 copies/50 ng DNA) were determined using a qPCR assay. b Flow cytometry contour plots of CAR-T cell functional potency as measured by a CD107a degranulation assay. MC10029 CAR-T cells (characterized in panel A) from three different healthy donors were incubated with WT Nalm-6 or BAFF-R KO Nalm-6 at an E:T ratio of 2:1. Analysis was gated on CD8 + CAR-T cell populations and showed no difference between the batches (Supplementary Fig. 7f). Non-transduced T cells (Non-CAR-T) from the same donor were used as negative controls