Abstract
We extended our previous observations with other tumor models to study seven ovarian tumor cell lines—OVCAR3, OVCAR4, OVCAR8, SKOV3, Kuramochi, OAW28, and CaOV3. We found that NK cells targeted and killed poorly differentiated OVCAR8 and CAOV3; these two tumor lines express lower MHC-class I and higher CD44 surface receptors. OVCAR3 and OVCAR4 were more resistant to NK cell-mediated cytotoxicity, and SKOV3, Kuramochi and OAW28 had intermediate sensitivity to NK cell-mediated cytotoxicity, likely representing well-differentiated and moderately differentiated ovarian tumor cell lines, respectively. Similar trends were observed for secretion of IFN-γ by the NK cells when co-cultured with different ovarian tumor cell lines. Treatment with both IFN-γ and TNF-α upregulated MHC-class I in all ovarian tumor cell lines and resulted in tumor resistance to NK cell-mediated cytotoxicity and decreased secretion of IFN-γ in co-cultures of NK cells with tumors cells with the exception of OVCAR8 and CAOV3 which did not upregulate MHC-class I and remained sensitive to NK cell-mediated cytotoxicity and increased secretion of IFN-γ when co-cultured with NK cells. Similarly, treatment with NK cell supernatants induced resistance to NK cell-mediated cytotoxicity in OVCAR4 but not in OVCAR8, and the resistance to killing was correlated with the increased surface expression of MHC-class I in OVCAR4 but not in OVCAR8. In addition, OVCAR4 was found to be carboplatin sensitive before and after treatment with IFN-γ and NK cell supernatants, whereas OVCAR8 remained carboplatin resistant with and without treatment with IFN-γ and NK cell supernatants. Overall, sensitivity to NK cell-mediated killing correlated with the levels of tumor differentiation and aggressiveness, and more importantly, poorly differentiated ovarian tumors were unable to upregulate MHC-class I under the activating conditions for MHC-class I, a feature that was not seen in other tumor models and may likely be specific to ovarian tumors. Such tumors may also pose a significant challenge in elimination by the T cells; however, NK cells are capable of targeting such tumors and can be exploited to eliminate these tumors in immunotherapeutic strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03192-7.
Keywords: Ovarian cancer, IFN-γ, TNF-α, Cytotoxicity, Differentiation, Chemotherapeutic drugs
Introduction
Ovarian cancer is the most lethal gynecologic cancer in the western world and is among the top five leading causes of death due to cancer in the United States of America [1, 2]. Due to the lack of reliable early detection methods, the majority of ovarian tumors go undetected until later stages. Unfortunately, advanced disease in this setting is difficult to treat. Despite the administration of treatments including systemic platinum-based chemotherapy and surgery, mortality rates for ovarian cancer have not changed in recent decades [1].
Natural killer (NK) cells are innate immune cells, representing approximately 5–20% of total lymphocytes in human peripheral blood, and are known for their anticancer function. NK cells are identified by CD16 and CD56 surface receptors, and are activated by a number of different cytokines [3, 4]. We have previously shown that NK cells limit the survival and expansion of cancer stem cells (CSCs)/poorly differentiated tumors via direct killing or induced differentiation through IFN-γ and TNF-α production [5]. These two mechanisms are indispensable for the effective targeting of tumor cells by the NK cells. IFN-γ and TNF-α secreted by the NK cells play a crucial role in the differentiation of CSCs, leading to increased expression of CD54 and MHC-class I and decreased levels of NK cell-mediated cytotoxicity against these NK-differentiated CSCs [6, 7]. However, NK cells are less capable of eliminating differentiated tumors those expressing higher levels of MHC-class I surface receptors [5]. Active receptors and co-receptors which recognize ligands on the tumor cells' surface induce NK cell activation [4, 5]. The diminished function of NK cells is linked to poor prognosis of cancer patients [8–18].
To understand which ovarian tumor cells are targeted by the NK cells and how NK cells discriminated between different ovarian tumors, we chose to study seven ovarian tumor cell lines. In this paper, we describe three different phenotypes of ovarian tumors with varying susceptibilities to NK cell-mediated cytotoxicity, which is likely dependent on the stages of differentiation in ovarian tumors. The following ovarian tumor cell lines were used in this study: OVCAR3, OVCAR4, OVCAR8, SKOV3, Kuramochi, CaOV3, and OAW28 [1, 2, 19–32].
In this study, we described a unique phenotype of ovarian tumors with the inability to upregulate MHC-class I under a number of activating conditions; however, these tumors remain susceptible to NK cell-mediated cytotoxicity, even though they are highly resistant to platin-mediated cell death and are likely at a poorly differentiated state. In addition, we described the phenotype of NK resistant ovarian tumors likely demonstrating a differentiated phenotype with higher sensitivity to platin-mediated killing, whereas intermediate sensitivity to NK cell-mediated cytotoxicity correlates with moderately differentiated ovarian tumors.
Results
Characterization of ovarian cancer cell lines based on MHC-class I and CD44 surface expression
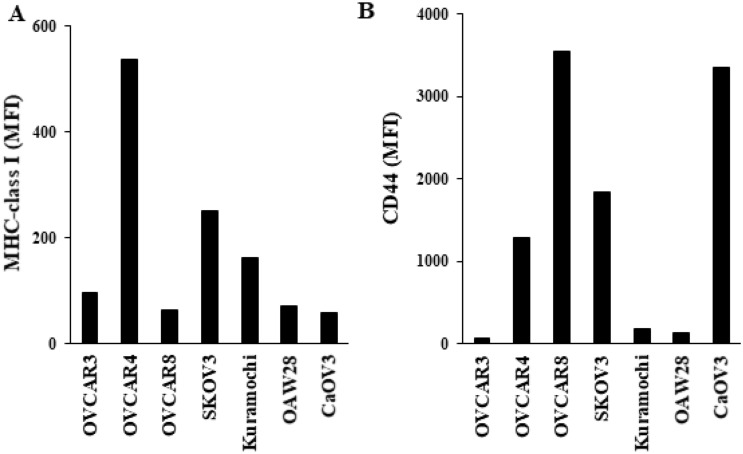
We determined the surface expression levels of MHC-class I and CD44 in seven ovarian cancer cell lines—OVCAR3, OVCAR4, OVCAR8, SKOV3, Kuramochi, OAW28, and CaOV3. We have previously demonstrated that cancer stem-like cells (CSCs)/poorly differentiated tumors exhibit higher CD44 and lower MHC-class I, whereas well-differentiated tumors exhibit decreased CD44 and increased MHC-class I surface express levels [5, 33, 34]. For MHC-class I, we observed the highest surface expression levels in OVCAR4 and lowest surface expression levels in OVCAR8 and CaOV3 (Fig. 1A). Other cell lines used in this study exhibited the following profiles: SKOV3 > Kuramochi > OVCAR3 > OAW28 for MHC-class surface expression levels (Fig. 1A). For CD44, we found the highest surface expression levels in OVCAR8 and CaOV3 and lowest surface expression levels in OVCAR3, and the following profile was seen in other cell lines SKOV3 > OVCAR4 > Kuramochi > OAW28 (Fig. 1B). These results indicated that OVCAR8 and CaOV3 exhibit CSCs like surface phenotype, whereas OVCAR4 exhibits differentiated tumors surface phenotype.
Fig. 1.
Surface expression of MHC-class I and CD44 on ovarian cancer cell lines. The surface expressions of MHC-class I (A) and CD44 (B) were assessed on ovarian cancer cell lines using flow cytometric analysis. IgG2 isotype control antibodies were used as controls. Mean fluorescence intensity (MFI) is shown in the figures. One of three representative experiments are shown in these figures
IFN-γ and TNF-α mediated modulation of MHC-class I and CD44 surface expression on ovarian cancer cell lines
We have previously demonstrated that IFN-γ and TNF-α mediate differentiation of a number of different CSCs/poorly differentiated tumors [7, 35]. Therefore, we used rh-IFN-γ and rh-TNF-α to induce differentiation in ovarian tumor cell lines (Fig. 2A). We found increased MHC-class I surface expression levels in ovarian cancer cell lines with rh-IFN-γ and rh-TNF-α treatments, except in OVCAR8 and CaOV3 (Fig. 2B, C, and S1A). We observed that rh-IFN-γ alone induced higher differentiation in comparison to rh-TNF-α alone, and the combination of rh-IFN-γ and rh-TNF-α induced highest differentiation (Fig. 2B, C). The surface expression level of CD44 was not much modulated with the treatment of rh-IFN-γ and rh-TNF-α (Figs. 2D and S1B). These results validated CSCs like phenotype of OVACR8 and CaOV3.
Fig. 2.
Surface expression of MHC-class I and CD44 after treatment of ovarian cancer cell lines with IFN-γ and/or TNF-α. Ovarian cancer cell lines (2 × 105 cells/well) were treated with IFN-γ (20 ng/ml), TNF-α (20 ng/ml), or a combination of IFN-γ (20 ng/ml) and TNF-α (20 ng/ml) for 18–20 h, before the surface expressions of MHC-class I and CD44 were assessed using flow cytometric analysis (A, B, D). IgG2 isotype control antibodies were used as controls. Fold change of MHC-class I mean fluorescence intensity (MFI) induced by IFN-γ (20 ng/ml), TNF-α (20 ng/ml), or the combination of IFN-γ (20 ng/ml) and TNF-α (20 ng/ml) treatments were determined in comparison to untreated cell lines (C). One of three representative experiments is shown in these figures
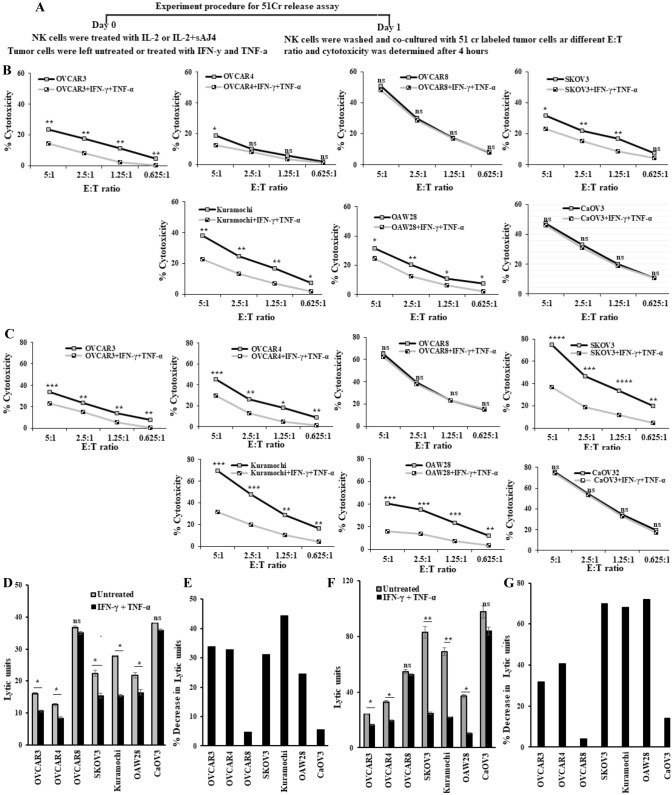
IFN-γ and TNF-α treatment mediated decreased susceptibility to NK cell-mediated cytotoxicity in ovarian cancer cell lines except in OVCAR8 and CaOV3
Our previous studies have demonstrated that CSCs/poorly differentiated tumors are excellent targets, whereas differentiated tumors are resistant to NK cell-mediated cytotoxicity [7, 36–38]. Here, we evaluated NK cell-mediated cytotoxicity against untreated and IFN-γ + TNF-α-treated ovarian tumors using IL-2 alone (Fig. 3A, B, D, E), and IL-2 + sAJ4 (Figs. 3A, C, F, G and S2) treated NK cells as effectors. AJ4 is a combination of Gram-positive probiotic bacteria strains; Streptococcus thermophiles, Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus paracasei. These probiotic bacteria strains were selected based on their superior ability to induce optimal and balanced secretion of both pro-inflammatory and anti-inflammatory cytokines from the NK cells [39–41]. Treatments of IFN-γ + TNF-α resulted in decreased susceptibility to NK cell-mediated cytotoxicity, except in OVACR8 and CaOV3 where slight/no change was seen (Figs. 3B–G, and S2). Based on susceptibility to NK cell-mediated cytotoxicity profile, OVACR8 and CaOV3 represent CSCs’ characteristics.
Fig. 3.
Resistance to NK cell-mediated cytotoxicity after treatment of ovarian cancer cell lines with IFN-γ and TNF-α. Ovarian cancer cell lines (2 × 105 cells/well) were treated with a combination of IFN-γ (20 ng/ml) and TNF-α (20 ng/ml) for 18–20 h, after which they were washed to remove unbound IFN-γ and TNF-α. NK cells purified from healthy individuals were treated with IL-2 (1000 U/ml) (A, B, D, E), or treated with a combination of IL-2 (1000 U/ml) and sAJ4 (20:1 bacteria to NK) (A, C, F, G) for 18 h before they were used as effectors against 51Cr-labeled untreated or IFN-γ- and TNF-α-treated ovarian cancer cell lines. Percentage cytotoxicity at various effector-to-target ratios was measured (B, C) and the lytic units (LU) 30/106 cells were determined using the inverse number of NK cells required to lyse 30% of target cells × 100 (D, F). Percentage decrease in lytic units 30/106 cells induced by IFN-γ + TNF-α treatments in comparison to untreated cell lines was determined (E, G). One of three representative experiments is shown in these figures
Supercharged NK cell supernatant mediated modulation of MHC-class I and CD54 surface expression, and resistance or susceptibility to NK cell-mediated cytotoxicity in OVCAR4 and OVACR8, respectively
Next, we treated OVCAR4 and OVCAR8 with supernatants harvested from osteoclasts-induced expanded NK cells (Fig. 4A). We have previously reported and patented a novel strategy to expand NK cells using osteoclasts as feeder cells, since these cells provide a number of important NK activating ligands in addition to the combination of key cytokines resulting in significant proliferation/expansion of NK cells with superior cytotoxicity and increased secretion of IFN-γ coined as supercharged NK cells [40, 42]. Supercharged NK cells were generated as described in the “Materials and methods” section. Increased surface expressions of CD54 and MHC-class I were seen on OVCAR4 both with the treatments with IFN-γ + TNF-α and with supercharged NK cell supernatants (Figs. 4B, S3A, S3C). In OVCAR8, with both treatments increased CD54 but not increased MHC-class I surface expressions were found (Figs. 4B, S3B, S3D). We have previously shown that NK cells mediate increase in the expression of CD54 and MHC-class I on differentiated tumor cells [7]. Decreased susceptibility to NK cell-mediated cytotoxicity was seen with OVCAR4, whereas a slight increase in OVCAR8 susceptibility was noted when these tumors were treated with supernatants of supercharged NK cells (Figs. 4C, S3E, S3F).
Fig. 4.
Surface expression of MHC-class I and CD54, and resistance or susceptibility to NK cell-mediated cytotoxicity of OVCAR4 and OVCAR8 when they were treated with supercharged NK cell supernatant, respectively. OVACR4 and OVCAR8 (2 × 105 cells/well) were treated with a combination of IFN-γ (20 ng/ml) and TNF-α (20 ng/ml), and also with the supernatants harvested from supercharged NK (sNK) cells as described in the “Materials and methods” section for 18–20 h before the surface expression of MHC-class I and CD54 were assessed using flow cytometric analysis. IgG2 isotype control antibodies were used as controls (A, B). OVCAR4 and OVCAR8 were treated with the supernatants harvested from supercharged NK (sNK) cells as described in the section “Materials and methods”. Super charged NK cells were treated with IL-2 (1000 U/ml) for 18 h before they were used as effectors against 51Cr-labeled sNK-supernatant-treated ovarian cancer cell lines. The lytic units (LU) were determined as described in Fig. 3C (C). One of three representative experiments is shown in these figures
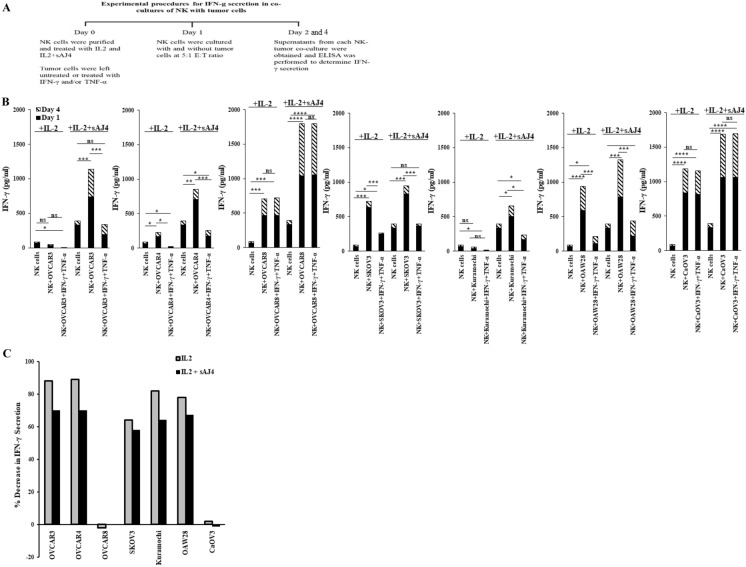
Unlike other ovarian tumors, secretion of IFN-γ remained high in co-cultures of NK cells with IFN-γ- and TNF-γ-treated OVCAR8 or CaOV3
Our laboratory has previously demonstrated that NK cells secrete higher levels of IFN-γ when co-cultured with CSCs/poorly differentiated tumors in comparison to differentiated tumors [7, 35]. In the current study, we co-cultured untreated and IFN-γ + TNF-α-treated ovarian tumors with IL-2 alone and IL-2 + sAJ4-treated NK cells (Fig. 5A). Decreased secretion of IFN-γ was found when IFN-γ + TNF-α-treated ovarian tumors were co-cultured with IL-2 alone or IL-2 + sAJ4-treated NK cells with the exception of those which were cultured with OVCAR8 and CaOV3 (Figs. 5B, C, and S4).
Fig. 5.
Secretion of IFN-γ when NK cells were co-cultured with IFN-γ- and TNF-α-treated ovarian cancer cell lines. Ovarian cancer cell lines, and NK cells were treated as described in Fig. 3. Ovarian cancer cell lines were washed to remove unbound IFN-γ and TNF-α, and were co-cultured with NK cells (NK:tumors; 5:1). The supernatants were harvested on days 1 and 4, and the levels of IFN-γ secretion were determined using specific ELISA (A, B). Percentage decrease of IFN-γ secretion induced by IFN-γ + TNF-α treatments in comparison to untreated cell lines was determined (C). One of three representative experiments is shown in these figures
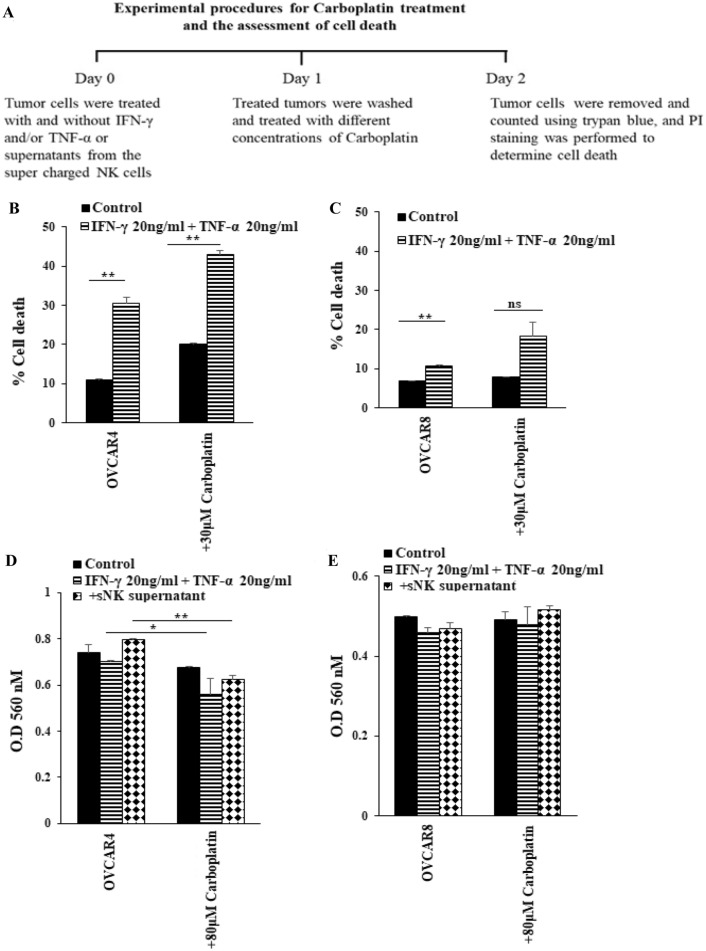
Treatment with either IFN-γ and TNF-α or supernatants from supercharged NK cells increased susceptibility to carboplatin-mediated killing in OVCAR4 but not in OVCAR8
It has been shown that differentiated tumors are more sensitive to chemotherapeutic drugs in comparison to CSCs/poorly differentiated tumors [34]. Carboplatin is a second-generation platinum compound with a broad spectrum of antineoplastic properties. Carboplatin is activated intracellularly to form reactive platinum complexes thereby inducing DNA and DNA–protein cross-links, resulting in apoptosis and cell growth inhibition [43]. We left ovarian cancer cell lines untreated or treated them with rh-IFN-γ + rh-TNF-α followed by carboplatin treatment before cell viability was determined (Figs. 6A–C, S5). Higher cell death was seen in OVCAR4 vs. OVCAR8 after carboplatin treatment both in the absence or presence of rh-IFN-γ + rh-TNF-α treatment (Fig. 6B, C). Decreased cell counts were obtained when tumors were treated with rh-IFN-γ + rh-TNF-α followed by carboplatin treatments in most tumors with the exception of OVCAR8 and CaOV3 (Figs. S5A, S5B). In addition, carboplatin induced highest cell death in rh-IFN-γ + rh-TNF-α-treated tumors with the exception of OVCAR8, OAW28, and CaOV3 which lower cell death were noted (Figs. S5C, S5D). The results with MTT were similar to those obtained with PI stained tumor cells treated with rh-IFN-γ + rh-TNF-α followed by carboplatin treatment (Fig. 6D, E). In addition, treatment of tumor cells with supernatants from supercharged NK cells followed by carboplatin treatment exhibited similar profiles to those seen when treated with rh-IFN-γ + rh-TNF-α (Fig. 6D, E).
Fig. 6.
Increased susceptibility of OVCAR4 but not OVCAR8 to carboplatin-mediated killing after treatment with IFN-γ and TNF-α. OVACR8 and OVCAR4 (2 × 105 cells/well) were treated with a combination of IFN-γ (20 ng/ml) and TNF-α (20 ng/ml). After an overnight incubation, cells were washed to remove unbound IFN-γ and TNF-α, and they were then treated with carboplatin (30 μM) for 18–20 h, after which the cells were stained with propidium iodine (PI) to determine percent cell death using flow cytometric analysis. One of three representative experiments is shown in this figure (A, B, C). The cells were left untreated or treated with the combination of TNF-α (20 ng/ml) and IFN-γ (20 ng/ml) or supernatants of sNKs’ cells (1:1 medium) for 24 h, after which the cells were washed or either left untreated or treated with carboplatin (80 µM) for an additional 24 h. Cell viability was determined using MTT assay (D, E). One of the three representative experiments is shown in this figure
Discussion
Ovarian cancer continues to be one of the most aggressive gynecological cancers. The goal of this report is to delineate the underlying mechanisms by which NK cells are able to target the ovarian tumors to limit or halt their progression. We also report on the role of NK cells in differentiation of ovarian tumors by NK supernatants and their subsequent resistance to NK cell-mediated cytotoxicity. Seven ovarian tumor cell lines were used for assessments, and three different phenotypes were established depending on susceptibility to NK cell-mediated cytotoxicity based on our previous studies [7, 33, 38, 44]. NK cells were found to target the poorly differentiated OVCAR8 and CAOV3 more than other tumor lines with different degrees of differentiation based on susceptibility to NK cell-mediated cytotoxicity. There was a great correlation between the levels of MHC-class I expression and targeting by the NK cells. Indeed, both OVCAR8 and CAOV3 had minimal expression of MHC-class I when compared to the other tumor lines. These two tumor lines expressed higher levels of CD44 which are one of the hallmarks of CSCs/poorly differentiated tumors. Although a significant correlation could not be seen by CD44 alone for different tumor lines, the combination of CD44 and MHC-class I was a good predictor of cellular susceptibility to NK cell-mediated killing and the potential levels of differentiation in different tumor lines.
NK cells limit tumor expansion by direct targeting and killing of the tumor cells, as well as through differentiation of the tumor cells by the secretion of IFN-γ and TNF-α [5]. These two mechanisms are the cornerstone of NK cell-mediated targeting of tumor cells, the former being specific to NK cells, whereas the latter could also be mediated by the activated T cells too. To determine whether treatment with IFN-γ and TNF-α is capable of differentiating the tumor cells, thereby decreasing NK cell-mediated cytotoxicity and secretion of IFN-γ, we determined the levels of MHC-class I expression and correlated to NK cell-mediated cytotoxicity and secretion of IFN-γ. As shown in Figs. 2 and S1, IFN-γ and TNF-α were capable of increasing MHC-class I expression in most tumors, albeit at differing levels, with the exception of OVCAR8 and CAOV3 in which regardless of how much IFN-γ and TNF-α were added to the tumors they did not upregulate the expression of MHC-class I, and they remained equally susceptible to NK cell-mediated cytotoxicity, whereas other tumor lines exhibited decreased levels of NK cell-mediated cytotoxicity after treatment with IFN-γ and TNF-α correlating with the degree of differentiation of the cells. Since supercharged NK cells augment secretion of IFN-γ and TNF-α, we also tested the increase in MHC-class I expression and susceptibility to NK cells in two tumor lines of OVCAR4 and OVCAR8 representing the two different spectrums of differentiation, the former being more differentiated and the latter being poorly or less differentiated phenotype. Treatment of OVCAR4 with NK supernatants upregulated MHC-class I significantly and resulted in the decrease in NK cell-mediated cytotoxicity, whereas OVCAR8 did not change the levels of MHC-class I and remained highly susceptible to NK cell-mediated cytotoxicity even after treatment with NK supernatants. These experiments suggested that ovarian tumors may become resistant to T-cell-mediated lysis due to the lack of upregulation of MHC-class I, whereas they may remain susceptible to NK cell-mediated effects. However, since many of the patients with ovarian tumors have also lower NK cell function, such tumors may persist and expand and result in the invasion and metastasis of the tumors [45, 46]. Increase in MHC-class I expression in these tumors may be one strategy by which T cells will be able to eliminate these tumors; however, it remains to be seen what treatment strategy could be able to increase the expression of MHC-class I in OVCAR8 and CAOV3. Whether over-expression of MHC-class I by genetic manipulation may result in the targeting of OVCAR8 and CAOV3 by T cells in the presence of a substantial decrease in NK cell-mediated cytotoxicity should await future studies. In addition, other cytokines secreted by the NK cells such as IFN-α, IL-1α, and TNF-β were also shown to increase MHC-class I expression, and therefore, may be able to increase expression on OVCAR8 and CAOV3 [47]. However, blocking with anti-IFN-γ and anti-TNF-α antibodies were found to substantially decrease the NK-induced expression of MHC-class I on other tumor models, indicating that IFN-γ and to a lesser degree TNF-α were the most dominant cytokines secreted by the NK cells were responsible for the upregulation of MHC-class I on tumor cells [7].
In our previous manuscript, NK cells were treated with monensin which is a Golgi-block immediately before their activation, and the results were compared to non-monensin-treated activated NK cells. The findings demonstrated that monensin blocked IFN-γ secretion substantially in NK cells, and inhibited the differentiation of the tumors and blocked MHC-class I upregulation on tumor cells resulting in the lack of induction of resistance in NK cell-mediated cytotoxicity, whereas those without monensin secreted very high levels of IFN-γ, and the secreted IFN-γ by the NK cell-mediated differentiation of the tumor cells leading to the upregulation of MHC-class I and induction of resistance of tumor cells to NK cell-mediated cytotoxicity [7].
We have previously shown that differentiated oral and pancreatic tumors were more susceptible to chemotherapeutic and radiotherapeutic strategies when compared to CSCs/poorly differentiated tumors [34]. Although clear differences could be seen in susceptibility of differentiated ovarian tumors to carboplatin-mediated decrease in tumor growth when treated with IFN-γ and TNF-α, OVCAR8 and CAOV3 were much less susceptible and the levels remained similar before and after treatment with IFN-γ and TNF-α. There were variable levels of susceptibility to carboplatin in other tumor types exhibiting the highest in OVCAR3 and OVCAR4 and lower in SKOV3, Kuramochi, and OAW28. Platinum drugs by binding to DNA form DNA adducts leading to the activation of apoptotic pathways. By reduction of intracellular drug concentration and/or changes in DNA repair mechanisms or the modification of cellular responses, tumor cells were shown to become resistant to carboplatin effect [48, 49]. Indeed, we have previously shown that differentiated oral tumors have lower expression of CD338 which is a member of the ATP-binding cassette transporter superfamily, and is known to contribute to multidrug resistance in cancer chemotherapy, and therefore, they were found to be more sensitive to cisplatin-mediated cell death, whereas their cancer stem cells/poorly differentiated tumors express much higher levels of CD338 and are resistant to cisplatin-mediated cell death [7, 34]. Whether such differences exist in ovarian tumors requires further investigation.
At present, the exact mechanisms by which certain ovarian tumor cell lines are able to upregulate MHC-class I, whereas the others lack such capability is not well understood, but it could be at the transcriptional, post-transcriptional, or translational levels [50]. We speculate that a number of mechanisms may be operational. It is possible that OVCAR8 and CAOV3 do not express adequate levels of IFN-γ and TNF-α receptors due to shedding or internalization of the receptors, and therefore, they do not respond to the secreted cytokines. Alternatively, or in addition, there may be multiple defects in the pathway of assembly and expression of MHC-class I on the surface of the tumors. Indeed, any mutations in the chaperone proteins or TAP or the peptide generations by the proteasomes could affect the stabilization and expression of MHC-class I on the surface of the tumor cells [50]. Epigenetic and post-transcriptional dysregulations of NFkB, IRFs, and NLRC5 can also be responsible for MHC-class I downregulation in ovarian cancer [50]. We hope to delineate these mechanisms in our future studies.
If NK cell function is not restored in ovarian cancer patients, chances are that the tumors with no or lower MHC-class I expression may escape T-cell-mediated cytotoxicity and expand and invade other tissues. Therefore, combined targeting of ovarian tumors by both competent NK cells as well as CD8 + T cells is paramount for successful treatment of these tumors. Our assessment of ovarian patient NK cells has shown that in the majority of patients, the function of NK cells are compromised (manuscript in prep). Therefore, to eliminate the aggressive ovarian tumors, it is absolutely necessary to restore and increase NK function in these patients. In addition, although IFN-γ and TNF-α secreted by the NK cells may not be able to increase MHC-class I expression on tumors similar to OVCAR8 or CAOV3, they will, however, be able to target and kill these tumors. Indeed, NK supernatant-treated OVCAR8 was found to exhibit increased NK cell-mediated cytotoxicity which was the complete opposite of OVCAR4 which it showed a decrease in NK cell-mediated cytotoxicity after treatment with NK cell supernatants.
There are several unresolved questions which will be studied in our future studies. For example, whether over-expression of MHC-class I on OVCAR8 or CAOV3 will render these cells resistant to NK cell-mediated cytotoxicity. Are there activators other than IFN-γ and TNF-α or supernatants from NK cells which are capable of upregulating MHC-class I on OVCAR8 or CAOV3? What strategies can be used to completely eliminate these tumors by the NK cells? Despite remaining questions our paper is significant, since we identified and characterized unique ovarian tumor phenotypes with the lack of ability to upregulate MHC-class I and their increased susceptibility to NK cell-mediated cytotoxicity. These tumors are quite different from other tumor models and those of the ovarian tumor cells which are able to upregulate MHC-class I under differentiation conditions, and, therefore, provide important tools for the future studies to determine how to eliminate such tumors in patients using NK immunotherapeutic strategies.
Materials and methods
Cell lines, reagents, and antibodies
Human NK cells were cultured in RPMI 1640 (Invitrogen by Life Technologies, CA), supplemented with 10% fetal bovine serum (FBS) (Gemini Bio-Products, CA). Seven Ovarian cancer cell lines—OVCAR3, OVCAR4, OVCAR8, SKOV3, Kuramochi, OAW28, and CaOV3—were purchased from ATCC or obtained from NIH under MTA. OVCAR3 and OVCAR4 were cultured in RPMI1640 supplemented with 10% FBS. OVCAR8, SKOV3, Kuramochi, OAW28, and CaOV3 were cultured in DMEM supplemented with 10% FBS. Recombinant IL-2 was obtained from NIH-BRB. Antibodies which were used for flow cytometry—IgG2, CD44, MHC-class I, and CD16—were purchased from Biolegend (San Diego, CA). Human NK cell purification kits were obtained from Stem Cell Technologies (Vancouver, BC, Canada).
Bacteria sonication
AJ4 is a combination of four different strains of Gram-positive probiotic bacteria (Streptococcus thermophiles, Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus paracasei). AJ2 is a combination of eight different strains of Gram-positive probiotic bacteria (Streptococcus thermophiles, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus bulgaricus). Both AJ2 and AJ4 were weighed and resuspended in RPMI 1640 containing 10% FBS at a concentration of 10 mg/1 mL. The bacteria were thoroughly vortexed and then sonicated on ice for 15 s, set at a 60% amplitude. Sonicated samples were then incubated for 30 s on ice. After every five pulses, a sample was taken to observe under the microscope until at least 80% of cell walls were lysed. It was determined that approximated 20 rounds of sonication/incubation on ice were conducted to achieve complete sonication. Finally, the sonicated samples (sAJ4 and sAJ2) were aliquoted and stored in − 80 °C freezer.
Purification of NK cells and monocytes from the peripheral blood
Written informed consents, approved by UCLA Institutional Review Board (IRB), were obtained from healthy individuals, and all procedures were approved by the UCLA-IRB. Peripheral blood was separated using Ficoll–Hypaque centrifugation, after which the white, cloudy layer containing peripheral blood mononuclear cells (PBMCs) was harvested. NK cells and monocytes were negatively selected from PBMCs using the EasySep® Human NK cell enrichment and EasySep® Human Monocytes enrichment kits, respectively, purchased from Stem Cell Technologies (Vancouver, BC, Canada). Purified NK cells and monocytes were stained with anti-CD16 and anti-CD14 antibodies, respectively, to measure purity using flow cytometric analysis. Samples showing greater than 95% purity were used for study.
Supernatant collection of supercharged NK cells
Monocytes were cultured in alpha-MEM media supplemented with M-CSF (25 ng/mL) for 21 days and RANKL (25 ng/mL) from day 6 to 21 days to generate osteoclasts (OCs). The media were replenished every 3 days. For NK cell expansion, purified NK cells were activated with rh-IL-2 (1000 U/ml) and anti-CD16 mAb (3 µg/ml) for 18–20 h before they were co-cultured with OCs and sAJ2 (OCs:NK:sAJ2; 1:2:4) in RPMI 1640 medium-containing 10% FBS. The media were refreshed every 3 days with RPMI complete medium-containing rh-IL-2 (1500 U/ml). The supernatant was harvested on day 12, and was used for ovarian cell line treatment.
Ovarian cell line differentiation and carboplatin treatments
Recombinant human interferon gamma (rhIFN-γ) and recombinant human tumor necrosis factor alpha (rhTNF-α) were purchased from PeproTech (Rocky Hill, NJ). Ovarian cancer cells were treated with rhIFN-γ (20 ng/ml) and rhTNF-α (20 ng/ml) or combination of rhIFN-γ (20 ng/ml) + rhTNF-α (20 ng/ml) for overnight to induce differentiation. In separate experiment, the supernatant of supercharged NK cells was used to induce differentiation in ovarian cancer cell lines. For carboplatin treatment, after an overnight incubation with rhIFN-γ (20 ng/ml) and rhTNF-α (20 ng/ml) or combination of rhIFN-γ (20 ng/ml) + rhTNF-α (20 ng/ml), samples were treated with carboplatin (10 μM/ml and 30 μM/ml) for overnight. The trypan blue staining was used to distinguish viable and non-viable cells, and the percentage of dead cells was determined propidium iodine (PI) (100 μg/ml) staining using flow cytometric analysis.
Surface staining analysis
Staining was performed by labeling the cells with antibodies as described previously [37, 51, 52]. Flow cytometric analysis was performed using Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, MA) and FlowJo v10.4 (BD, Oregon, USA) was used for analysis; Beckman Coulter Epics XL cytometer (Brea, CA), and results were analyzed in the FlowJo vX software (Ashland, OR).
Enzyme-Linked Immunosorbent Assays (ELISAs)
Single ELISAs were performed as previously described [37]. To analyze and obtain the cytokine and chemokine concentration, a standard curve was generated by either two- or three-fold dilutions of recombinant cytokines provided by the manufacturer.
51Cr release cytotoxicity assay
The 51Cr release cytotoxicity assay was performed as previously described [53]. Briefly, different ratios NK cells and 51Cr-labeled ovarian cell lines were incubated for 4 h. After which, the supernatants were harvested from each sample, and the released radioactivity was counted using the gamma counter. The percentage specific cytotoxicity was calculated as follows:
LU 30/106 is calculated using the inverse of the number of NK cells needed to lyse 30% of ovarian cell lines × 100.
MTT assay
The ovarian tumors were grown to 80% confluency in a 96-well plate. After that, the media were changed, and the cells were left untreated or treated with the combination of rhTNF-α (20 ng/ml) and rhIFN-γ (50 ng/ml) or with the supernatants of sNKs’ cells (1:1 in medium) for 24 h. Thereafter, the cells were left untreated or treated with carboplatin (80 μM) for an additional 24 h, after which the cell viability assay was performed using cell proliferation kit (MTT) (Roche Diagnostics Co., Germany), following the manufacturer’s suggestions. Results were obtained using an ELISA plate reader Multiskan FC ELISA reader (Thermo Scientific, Waltham, MA).
Statistical analyses
An unpaired or paired, two-tailed Student’s t test was performed for experiments with two groups. One-way ANOVA with a Bonferroni post-test was used to compare different groups for experiments with more than two groups. Duplicate or triplicate samples were used for assessment. The following symbols represent the levels of statistical significance within each analysis: ***(p value < 0.001), **(p value 0.001–0.01), *(p value 0.01–0.05).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to funding agencies and donors for supporting the work.
Author contributions
NC, SHY, and PCC generated data, reviewed, and edited the article. KK analyzed data, prepared figures, wrote, reviewed, and edited the article. NA and GD provided tumor samples and technical help. SG reviewed and edited the paper. AJ and SM oversaw the studies, conceptualization of the article, reviewed and edited the article, and acquired funding.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nishant Chovatiya, Kawaljit Kaur, Sara Huerta-Yepez, and Po-Chun Chen have contributed equally to this work.
Contributor Information
Sanaz Memarzadeh, Email: smemarzadeh@mednet.ucla.edu.
Anahid Jewett, Email: ajewett@mednet.ucla.edu.
References
- 1.Beaufort CM, et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS ONE. 2014;9(9):e103988. doi: 10.1371/journal.pone.0103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haley J, et al. Functional characterization of a panel of high-grade serous ovarian cancer cell lines as representative experimental models of the disease. Oncotarget. 2016;7(22):32810–32820. doi: 10.18632/oncotarget.9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perera Molligoda Arachchige AS. Human NK cells: from development to effector functions. Innate Immun. 2021;27(3):212–229. doi: 10.1177/17534259211001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poli A, et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bui VT, et al. Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. 2015;6:576. doi: 10.3389/fimmu.2015.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Tseng HC, et al. Induction of split anergy conditions natural killer cells to promote differentiation of stem cells through cell-cell contact and secreted factors. Front Immunol. 2014;5:269. doi: 10.3389/fimmu.2014.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hersey P, et al. Low natural-killer-cell activity in familial melanoma patients and their relatives. Br J Cancer. 1979;40(1):113–122. doi: 10.1038/bjc.1979.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuss I, et al. Clinical significance of decreased zeta chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res. 1999;5(2):329–334. [PubMed] [Google Scholar]
- 10.Guillerey C. NK Cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1273:69–90. doi: 10.1007/978-3-030-49270-0_4. [DOI] [PubMed] [Google Scholar]
- 11.Lee JC, et al. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172(12):7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 12.Imai K, et al. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 13.Jewett A, et al. Rapid and potent induction of cell death and loss of NK cell cytotoxicity against oral tumors by F(ab')2 fragment of anti-CD16 antibody. Cancer Immunol Immunother. 2008;57(7):1053–1066. doi: 10.1007/s00262-007-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietra G, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72(6):1407–1415. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- 15.Hershey P, et al. Relationship of cell-mediated cytotoxicity against melanoma cells to prognosis in melanoma patients. Br J Cancer. 1978;37(4):505–513. doi: 10.1038/bjc.1978.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai P, et al. Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res. 1996;2(1):161–173. [PubMed] [Google Scholar]
- 17.Burke S, et al. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010;31(9):339–345. doi: 10.1016/j.it.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Brittenden J, et al. Natural killer cells and cancer. Cancer. 1996;77(7):1226–1243. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1226::AID-CNCR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Patankar MS, et al. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99(3):704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Gubbels JA, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubbels JA, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5(1):50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SW, et al. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997;57(5):850–856. [PubMed] [Google Scholar]
- 23.Mitra AK, et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol Oncol. 2015;138(2):372–377. doi: 10.1016/j.ygyno.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottschalk N, et al. Monocytes and the 38kDa-antigen of mycobacterium tuberculosis modulate natural killer cell activity and their cytolysis directed against ovarian cancer cell lines. BMC Cancer. 2012;12:451. doi: 10.1186/1471-2407-12-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, et al. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget. 2015;6(11):9313–9326. doi: 10.18632/oncotarget.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natoli M, et al. Human ovarian cancer intrinsic mechanisms regulate lymphocyte activation in response to immune checkpoint blockade. Cancer Immunol Immunother. 2020;69(8):1391–1401. doi: 10.1007/s00262-020-02544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, et al. Natural killer cells inhibit metastasis of ovarian carcinoma cells and show therapeutic effects in a murine model of ovarian cancer. Exp Ther Med. 2018;16(2):1071–1078. doi: 10.3892/etm.2018.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoogstad-van Evert JS, et al. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol Oncol. 2020;157(3):810–816. doi: 10.1016/j.ygyno.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez VD, et al. High-grade serous ovarian tumor cells modulate NK cell function to create an immune-tolerant microenvironment. bioRxiv. 2020;9:361. doi: 10.1016/j.celrep.2021.109632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannistra SA, et al. Functional heterogeneity of CD44 molecules in ovarian cancer cell lines. Clin Cancer Res. 1995;1(3):333–342. [PubMed] [Google Scholar]
- 32.Cannistra SA, et al. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53(16):3830–3838. [PubMed] [Google Scholar]
- 33.Kaur K, et al. Probiotic-treated super-charged NK cells efficiently clear poorly differentiated pancreatic tumors in Hu-BLT mice. Cancers (Basel) 2019;12(1):63. doi: 10.3390/cancers12010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozlowska AK, et al. Differentiation by NK cells is a prerequisite for effective targeting of cancer stem cells/poorly differentiated tumors by chemopreventive and chemotherapeutic drugs. J Cancer. 2017;8(4):537–554. doi: 10.7150/jca.15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bui VT, et al. Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. 2015 doi: 10.3389/fimmu.2015.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jewett A, Man Y-G, Tseng H-C. Dual functions of natural killer cells in selection and differentiation of stem cells; role in regulation of inflammation and regeneration of tissues. J Cancer. 2013;4(1):12–24. doi: 10.7150/jca.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156(3):907–915. [PubMed] [Google Scholar]
- 38.Tseng H-C, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE. 2010;5(7):e11590. doi: 10.1371/journal.pone.0011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur K, et al. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology. 2018;7(5):e1426518. doi: 10.1080/2162402X.2018.1426518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur K, et al. Novel strategy to expand super-charged NK Cells with significant potential to lyse and differentiate cancer stem cells: differences in NK expansion and function between healthy and cancer patients. Front Immunol. 2017;8:297. doi: 10.3389/fimmu.2017.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bui VT, et al. Augmented IFN-gamma and TNF-alpha induced by probiotic bacteria in NK Cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol. 2015;6:576. doi: 10.3389/fimmu.2015.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng HC, et al. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: role in osteoclast-mediated NK cell activation. Oncotarget. 2015;6(24):20002–20025. doi: 10.18632/oncotarget.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagstaff AJ, et al. Carboplatin. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs. 1989;37(2):162–190. doi: 10.2165/00003495-198937020-00005. [DOI] [PubMed] [Google Scholar]
- 44.Jewett A, et al. Multiple defects of natural killer cells in cancer patients: anarchy, dysregulated systemic immunity, and immunosuppression in metastatic cancer. Crit Rev Immunol. 2020;40(2):93–133. doi: 10.1615/CritRevImmunol.2020033391. [DOI] [PubMed] [Google Scholar]
- 45.Nersesian S, et al. Naturally killing the silent killer: NK cell-based immunotherapy for ovarian cancer. Front Immunol. 2019;10:1782–1782. doi: 10.3389/fimmu.2019.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutgendorf SK, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23(28):7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Wicks IP, et al. The effect of cytokines on the expression of MHC antigens and ICAM-1 by normal and transformed synoviocytes. Autoimmunity. 1992;12(1):13–19. doi: 10.3109/08916939209146125. [DOI] [PubMed] [Google Scholar]
- 48.Galluzzi L, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 49.Halon A, et al. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res. 2011;31(2):711–718. [PubMed] [Google Scholar]
- 50.Cornel AM, Mimpen IL, Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers (Basel) 2020;12(7):1760. doi: 10.3390/cancers12071760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jewett A, Cavalcanti M, Bonavida B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J Immunol. 1997;159(10):4815–4822. [PubMed] [Google Scholar]
- 52.Jewett A, Bonavida B. Interferon-alpha activates cytotoxic function but inhibits interleukin-2-mediated proliferation and tumor necrosis factor-alpha secretion by immature human natural killer cells. J Clin Immunol. 1995;15(1):35–44. doi: 10.1007/BF01489488. [DOI] [PubMed] [Google Scholar]
- 53.Jewett A, et al. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor kappaB in HEp2 tumor cell line: effect on the function of natural killer cells. Hum Immunol. 2003;64(5):505–520. doi: 10.1016/S0198-8859(03)00039-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.