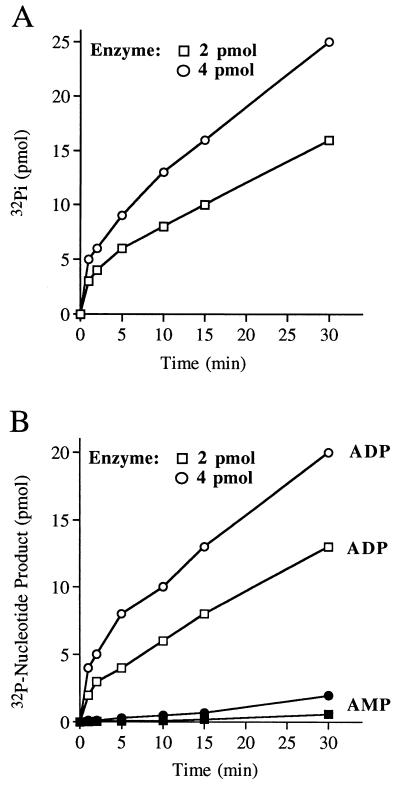
FIG. 6.
Kinetics of ATP hydrolysis by the 9.5S phosphatase. (A) Reaction mixtures containing (per 10 μl) 10 μM [γ-32P]ATP and either 2 or 4 pmol of 23-kDa protein from glycerol gradient fraction 6 were incubated at 30°C. Aliquots (10 μl) were withdrawn at 1, 2, 5, 10, 15, and 30 min and quenched immediately by mixing with 1 μl of 1 M formic acid. The reaction products were analyzed by PEI-cellulose TLC. The extent of 32Pi formation is plotted as function of reaction time. (B) Reaction mixtures containing (per 10 μl) 10 μM [α-32P]ATP and either 2 or 4 pmol of 23-kDa protein from fraction 6 were incubated at 30°C. The extents of ADP and AMP formation are plotted as function of reaction time.