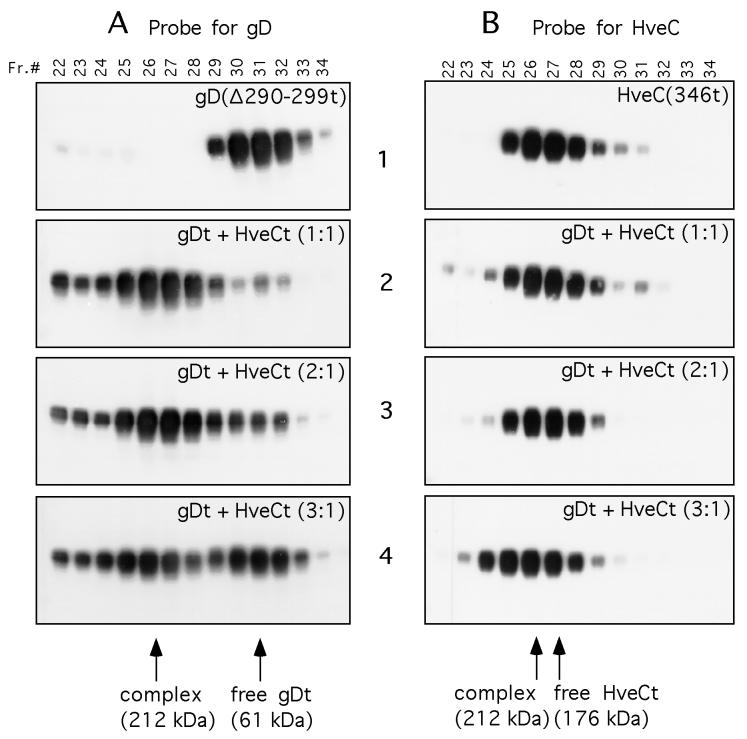
FIG. 9.
Gel filtration chromatography of the HveC-gD complex. Purified HveCt and gD(Δ290-299t) were loaded independently or mixed at the indicated ratio on a Superdex 200 column. Elution was performed with PBS and monitored by measuring UV absorption at 280 nm. Fractions (Fr.) of 0.5 ml were collected and analyzed by SDS-PAGE in denaturing and reducing conditions. After protein transfer, blots were probed with serum R7 to detect gD (A) or R145 to detect HveC (B). Sizes of complexes were calculated according to elution of standards used to calibrate the column. Purified HveCt (A1) or gD(Δ290-299t) (B1) was diluted to 20 μM in PBS and loaded on the column. Panels A2 and B2 show protein elution from a column loaded with gD(Δ290-299t) (20 μM) and HveCt (20 μM) premixed overnight at 4°C in PBS. The initial molar ratio of gDt monomer to HveCt monomer is 1:1. Panels A3 and B3 show protein elution from a column loaded with gD(Δ290-299t) (20 μM) and HveCt (10 μM) mixed overnight at 4°C in PBS. The initial molar ratio of gD to HveC is 2:1. Panels A4 and B4 show protein elution from a column loaded with gD(Δ290-299t) (30 μM) and HveCt (10 μM) mixed overnight at 4°C in PBS. The initial molar ratio of gD to HveC is 3:1.