Abstract
Cancer immunotherapy using immune checkpoint inhibitors (ICIs) has been recognized as a novel therapeutic option for head and neck squamous cell carcinoma (HNSCC). However, only approximately 20–30% of patients with recurrent/metastatic (R/M) HNSCC benefit. Moreover, the mechanisms underlying the response to ICIs remain unclear. We investigated the proportion, activation status, and expression level of immune checkpoint molecules in circulating T cell subsets in R/M HNSCC patients treated with nivolumab using flow cytometry and mass cytometry, and then determined whether treatment response was associated with these values. We also assessed the changes in the frequency of tumor-associated antigens, MAGE-A4 and p53, -specific T cells prior to and after nivolumab treatment using the IFN-γ ELISPOT assay. The proportion of activated CD4+ and CD8+ TEMRA cells significantly increased in the disease-controlled patients but not in disease-progressed patients. As expected, the expression of PD-1 in T cells markedly decreased regardless of the therapeutic response. Meanwhile, T cell immunoglobulin mucin-3 expression on CD8+ T cells was significantly higher in patients with disease progression than in disease-controlled patients after treatment. The frequency of the tumor-associated antigens, MAGE-A4- and p53-specific T cells, was not correlated with clinical responses; however, in the disease-controlled patients, the frequency of MAGE-A4-specific T cells was significantly augmented. We concluded that in R/M HNSCC patients treated with nivolumab, circulating T cells show dynamic alterations depending on treatment efficacy. An analysis of the immunokinetics of circulating T cells could thus provide new insights into rational therapeutic strategies in cancer immunotherapy for HNSCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-021-03042-y.
Keywords: Head and neck squamous cell carcinoma, Nivolumab, Treatment response, T lymphocyte
Introduction
In cancer patients, T cells possessing antitumor activity become exhausted in circulation as well as the tumor microenvironment, and cannot exert antitumor effector function through the upregulation of immune checkpoint molecules, including programmed cell death-1 (PD-1), lymphocyte activation gene 3 (Lag-3), and T cell immunoglobulin mucin-3 (Tim-3) [1, 2]. PD-1 is a major regulator of T cell exhaustion, and blockade of the PD-1/ programmed cell death-1 ligand 1 (PD-L1) axis reinvigorates exhausted T cells, leading to tumor regression [3, 4]. Cancer immunotherapy using immune checkpoint inhibitors (ICIs) has been recognized as a novel therapeutic option for various malignancies, including head and neck squamous cell carcinoma (HNSCC) [5–7]. However, only approximately 20–30% of patients with recurrent/metastatic (R/M) HNSCC benefit from ICI treatment. Furthermore, the mechanisms underlying the response to ICIs remain unclear. Tissue-based biomarkers, including PD-L1 expression on tumor cells and tumor-infiltrating immune cells, tumor mutation burden, CD8 T cell infiltration, and interferon (IFN)-γ gene signatures, have been extensively investigated [8–10]. However, tumor tissue sampling has several problems that are invasive, sometimes unrepeatable and inaccessible, and do not reflect comprehensive tumor profiling. When ICIs exert therapeutic effects, tumor-infiltrating tumor-specific T cells are activated and kill tumor cells, but also initiate or boost antitumor immune responses, implying a self-sustaining cancer immunity cycle [11]. Under these circumstances, circulating immune cells in peripheral blood can also serve as surrogate markers to evaluate antitumor immune responses in the tumor microenvironment; this is because the effector cells activated in lymph nodes enter the circulatory system, finally reaching the tumor sites and performing their function [12–14]. Moreover, peripheral blood samples are less invasive, easily accessible, and repeatable for analysis compared to tumor tissue. Kamphorst et al. revealed an increase in Ki-67+ PD-1+ CD8 T cells following PD-1-targeted therapy in 70% of lung cancer patients. Further, 80% of patients with clinical benefit exhibited PD-1+ CD8 T cell responses [15]. Similarly, high central memory T cell-to-effector memory ratios in the blood were found to correlate with longer progression-free survival in lung cancer patients receiving nivolumab [16]. In HNSCC, the percentage of PD-1+ CD8+ effector T cells was significantly lower in responders to nivolumab [17], indicating that the co-expression of immune checkpoint molecules on circulating T cells could be a predictor for the treatment efficacy of ICI therapy. Thus, evaluation of the distributional and functional alterations of peripheral immune cell subsets, particularly T cell subsets, may be useful as biomarker candidates for predicting ICI efficacy.
In the present study, we first investigated the proportion, activation status, and expression level of immune checkpoint molecules in circulating T cell subsets in R/M HNSCC patients treated with nivolumab. Thereafter, we determined whether the response to nivolumab treatment was associated with these values. Second, to gain a deeper understanding of longitudinal phenotypic alterations at the single-cell level, cytometry by time-of-flight (CyTOF) analysis was performed using representative samples obtained from eight patients receiving nivolumab treatment. Third, to evaluate dynamic changes in tumor-specific T cell responses, two tumor-associated antigens (TAAs) preferentially expressed in HNSCC [18, 19], melanoma-associated antigen-A4 (MAGE-A4), and p53 were selected, and the changes in frequency of TAA-specific T cells were analyzed. Elucidation of the relationship between dynamic changes in immune responses following ICI administration and treatment responses could lead to the identification of potential biomarkers in patients with R/M HNSCC as well as clarify the immune mechanisms underlying ICI response or resistance.
Materials and methods
Patients and blood collection
Patients with histologically confirmed R/M HNSCC who were treated with nivolumab were eligible for inclusion in this study. Treatment response was evaluated according to RECIST version 1.1. during weeks 8–10. If the patients had an unacceptable level of drug-related toxic effects or rapid disease progression before evaluation, the therapeutic effects were evaluated at that point.
Peripheral blood samples were obtained from 36 patients with R/M HNSCC treated with nivolumab, and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation and cryopreserved until used. If possible, blood samples were collected at two time points: before treatment initiation and after four infusions of nivolumab. This study was approved by the Ethical Committee of Gunma University Hospital (HS2017-152). Written informed consent was obtained from each patient.
Flow cytometry
Cryopreserved PBMCs were thawed, washed, and resuspended in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) and 0.1% NaN3. Non-specific binding of antibodies to Fc receptors on PBMCs was blocked using BD Fc Block (BD Bioscience, San Jose, CA, USA) in accordance with the manufacturer's instructions. Thereafter, PBMCs were stained with antibodies specific for CD3, CD4, CD8, CD25, CD38, CD45RA, CCR7, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), PD-1, lymphocyte activation gene 3 (Lag-3), Tim-3, forkhead box P3 (Foxp3), and Ki-67. The antibodies used in this study are listed in Supplementary Table 1. For intracellular staining of Foxp3 and Ki-67, the Foxp3 transcription factor fixation/permeabilization concentrate and diluent solutions (eBioscience, Santa Clara, CA) were used according to the manufacturer's instructions. As a negative control, the cells were stained with mouse IgG isotype control (BD Biosciences). Flow cytometry was conducted using a FACSVerse flow cytometer (BD Bioscience), and data analysis was performed using FlowJo software (TreeStar, Ashland, OR).
Mass cytometry
Mass cytometry was performed using the thawed PBMCs. Nonspecific binding of antibodies to the FC receptors was blocked using BD Fc Block (BD Bioscience). Surface antigen staining was performed using the Maxpar Basic Human T Cell Immuno-Oncology Panel Set (Fluidigm, South San Francisco, CA), which contains 24 metal isotope-conjugated antibodies (Supplementary Table 2), according to the manufacturer’s protocol. Stained cells were fixed overnight with 1% PFA. Fixed cells were stained with 250 nM Cell-ID Intercalator-Ir (Fluidigm) in permeabilization buffer for cell identification, and then washed with Maxpar Cell Acquisition Solution (Fluidigm). Thereafter, cells were resuspended in Maxpar Cell Acquisition Solution with a 1:10 dilution of EQ Four Element Calibration beads (Fluidigm). Samples were acquired on a Helios CyTOF mass cytometer (Fluidigm) at an event rate of 500 events/second or less.
Data analysis of mass cytometry results
FCS data files were bead-normalized using CyTOF software version 7.0.8493 (Fluidigm). Cell debris, dead cells, and doublets were excluded for the identification of live cells using FlowJo software (TreeStar, Ashland, OR, USA). CD45+ CD3+ cells were gated as T cells using FlowJo software. Further analysis was performed using R (The R Foundation for Statistical Computing, Vienna, Austria) in combination with R studio version 1.3.1093 (R studio, Boston, MA, USA) based on a previously described workflow [20]. In brief, the intensity of each marker was transformed using the arcsinh scale with cofactor 5. A multi-dimensional scaling (MDS) plot was constructed to demonstrate the similarities between samples using the plotMDS function from the limma package. Cell clustering was performed using the SOM function from the FlowSOM package and the ConsensusClusterPlus function from the ConsensusClusterPlus package. Uniform manifold approximation and projection (UMAP) for dimension reduction was used to visualize the data in a 2D map. A total of 5000 cells were randomly selected from each sample, and UMAP projection was performed using the umap package. Visualization was subsequently performed using the ggplot package.
Immunohistochemistry (IHC) and evaluation
Immunohistochemical detection of MAGE-A4 and p53 in tumor specimens was performed as previously described [21]. Briefly, formalin-fixed paraffin-embedded specimens were sectioned (3 µm thick) and deparaffinized. Antigen retrieval was achieved by boiling the samples at 98 °C for 30 min in citrate buffer (pH 6.0) and 98 °C for 30 min in 20% zinc sulfate solution for MAGE-A4 and p53 staining, respectively. After blocking, slides were incubated at room temperature for 2 h with primary antibodies (anti-MAGE-A4 antibody [1:2000], clone 57 B, MERCK; anti-p53 antibody [1:600], NCL-L-p53-DO7, NOVOCASTRA) and then incubated overnight at 4 °C. Subsequently, slides were incubated with a secondary antibody (Histofine Simple Stain MAX-PO (MULTI), Nichirei) and the reaction products were detected with 3,3’-diaminobenzidine (DAB, DOJINDO, Kumamoto, Japan). Sections were counterstained with Mayer’s hematoxylin.
Slides were assessed by two independent investigators (S.I. and K.C.) in a blinded fashion. For MAGE-A4, each specimen was positive if specific staining was present. For p53, a microscopic examination of nuclear staining was performed and specific staining in > 10% of the tumor cells was defined as positive expression, as described in a previous report [21].
In vitro sensitization and enzyme-linked immunosorbent spot assays
To evaluate the specific T cell responses of two TAAs, MAGE-A4 and p53, IFN-γ ELISPOT assays were performed as previously described [21]. Briefly, thawed PBMCs were cultured in the presence of recombinant protein (10 µg/mL of MAGE-A4 or p53) in a final volume of 1 mL AIM-V medium supplemented with 10 IU/mL interleukin (IL)-2 and 5 ng/mL IL-7 in a 24-well tissue culture plate. Recombinant MAGE-A4 and p53 proteins were purified using a nickel affinity column from transgenic silkworm silk glands expressing MAGE-A4 and TP53 constructs [21, 22]. After 4 days, 1 mL of AIM-V medium containing 10 IU/mL IL-2 was added to each well. Following 3 days of incubation, PBMCs were harvested, washed, and tested using IFN-γ ELISPOT assays.
ELISPOT assays were performed using a Human IFN-γ single-color ELISPOT kit (Cellular Technology Ltd., Cleveland, OH, USA) according to the manufacturer’s protocol. Effector cells (1–5 × 104 cells/well) were co-cultured with PBMCs (1 × 105 cells/well) in the presence of MAGE-A4 or p53 protein (10 µg/mL each) in a 96-well plate precoated with the IFN-γ capture antibody. PBMCs stimulated with 10 µg/mL phytohemagglutinin (PHA) were used as a positive control. The plates were incubated at 37 °C for 24 h. After incubation, the plates were washed and developed with anti-human IFN-γ (biotinylated) and streptavidin–alkaline phosphatase. The number of spot-forming cells (SFCs) in each well was automatically counted using a CTL-ImmunoSpot Analyzer (Cellular Technology Ltd.). The mean number of spots in control wells (no protein) was subtracted from the mean number of spots in the experimental wells, and the results were expressed as SFC per 5 × 104 cells.
Statistical analysis
GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA) was used to perform statistical analyses. The Mann–Whitney U test, Wilcoxon signed-rank test, and Fisher’s exact test were used to determine significant differences in the continuous and categorical variables. Two-sided P values < 0.05 were considered to be statistically significant.
Results
Patient characteristics
We analyzed 36 patients with R/M HNSCC who received the anti-PD-1 blocking antibody, nivolumab. A summary of the patient characteristics is shown in Table 1. The objective response and disease control rates were 22.2% and 41.6%, respectively. Ten patients discontinued 4 courses of nivolumab due to rapid disease progression or deteriorating conditions (n = 8) and intolerable immune-related adverse events (n = 2). Peripheral blood sample was collected from 24 (66.7%) of the 36 patients at two time points: before treatment initiation and after four infusions of nivolumab.
Table 1.
Demographic and clinical characteristics of HNSCC patients in this study
| Clinical variable | Total (%) | ||
|---|---|---|---|
| n = 36 | |||
| Age (years): median (range) | 66 (46–86) | ||
| Sex | Male | 31 (86.1) | |
| Female | 5 (13.9) | ||
| Primary site | Paranasal sinus | 7 (19.4) | |
| Oral cavity | 3 (8.3) | ||
| Nasopharynx | 2 (5.6) | ||
| Oropharynx | HPV-positive | 3 (8.3) | |
| HPV-negative | 3 (8.3) | ||
| Hypopharynx | 14 (38.9) | ||
| Larynx | 2 (5.6) | ||
| Parotid gland | 1 (2.8) | ||
| External auditory canal | 1 (2.8) | ||
| Local recurrence | (−) | 19 (52.8) | |
| (+) | 17 (47.2) | ||
| Lymph node metastasis | (−) | 16 (44.4) | |
| (+) | 20 (55.6) | ||
| Distant metastasis | (−) | 15 (41.7) | |
| (+) | 21 (58.3) | ||
| Nivolumab treatment | < 4 times | 10 (27.8) | |
| ≥ 4 times | 26 (72.2) | ||
| Treatment response | CR | 0 (0.0) | |
| PR | 8 (22.2) | ||
| SD | 7 (19.4) | ||
| PD | 21 (58.3) | ||
| TAA expression | MAGE-A4 | (−) | 15 (41.7) |
| (+) | 21 (58.3) | ||
| p53 | (−) | 12 (33.3) | |
| (+) | 24 (66.7) | ||
HNSCC: Head and neck squamous cell carcinoma, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, TAA: tumor-associated antigen
Characteristics of the T cell subsets and clinical response to nivolumab
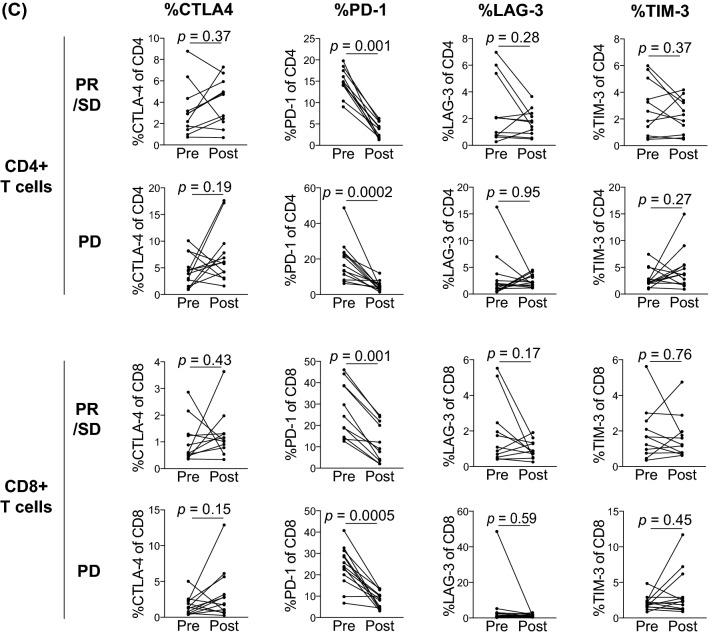
First, we used flow cytometry to determine the proportion of T cell subsets (naïve [TN]; CD45RA+ CCR7+, central memory [TCM]; CD45RA−CCR7+, effector memory [TEM]; CD45RA−CCR7−, and terminally differentiated effector memory (TEMRA); CD45RA+ CCR7−), activation status (CD38+ and Ki-67+), and expression of immune checkpoint molecules (CTLA-4, PD-1, Lag-3, and Tim-3) for CD4+ and CD8+ T cells in peripheral blood collected pre-treatment from R/M HNSCC patients administered nivolumab. The flow cytometry gating strategy used for the experiment is shown in Supplementary Fig. 1. Unfortunately, the proportions of each T cell subset, activated T cells, and immune checkpoint molecule-expressing T cells were not significantly different between the two groups (i.e., disease-controlled and disease-progressed patients) (Supplementary Fig. 2). Likewise, in post-treatment peripheral blood, the proportions of each T cell subset and activated T cells were not significantly different between the two groups, except for the proportion of CD4+ TEM cells (p = 0.04) (Fig. 1a and b). Meanwhile, the expression of two immune checkpoint molecules, Lag-3 and Tim-3, on CD8+ T cells in patients with disease progression was significantly higher than that in disease-controlled patients after nivolumab treatment, as shown in Fig. 1c. The expression of Tim-3 in CD4+ T cells also showed a similar tendency; however, the difference was not statistically significant (CD4+ T cells, Lag-3; p = 0.12, Tim-3; p = 0.05, CD8 + T cells, Lag-3; p = 0.04, Tim-3; p = 0.04).
Fig. 1.
Comparison of the proportions of T cell subset, activated T cells, and immune checkpoint molecule-expressing T cells in peripheral blood post-treatment based on the clinical response to nivolumab and analysis using flow cytometry. Peripheral blood samples of 24 R/M HNSCC patients treated with nivolumab were collected after 4 infusions of nivolumab. a The proportion of T cell subsets for each CD4+ and CD8+ T cells. b The proportion of CD38+ and Ki-67+ cells for each CD4+ and CD8+ T cells. c The proportion of the immune checkpoint molecules, CTLA-4, PD-1, Lag-3, and Tim-3 expressing T cells, for each CD4+ and CD8+ T cells
Alterations of the T cell subsets and clinical response to nivolumab
We examined the alterations in the proportion of T cell subsets, activated T cells, and immune checkpoint molecules expressing T cells for CD4+ and CD8+ T cells in the peripheral blood before and after nivolumab treatment. As shown in Fig. 2a, the proportion of CD4+ TEMRA was significantly increased in disease-controlled patients (p = 0.01), but not in patients with disease progression (p = 0.60). Meanwhile, the proportion of CD8+ TEMRA tended to increase in patients with disease control (p = 0.08). Of note, the proportion of activated CD4+ and CD8+ TEMRA T cells identified by CD38 expression was more clearly increased in disease-controlled patients (Fig. 2b, CD4+ TEMRA; p = 0.03, CD8+ TEMRA; p = 0.01). Moreover, the proportion of activated status was significantly increased in all CD8+ T cell subsets, except TEM in disease-controlled patients (CD8+ T cell; p = 0.02, CD8+ TN, p = 0.003, CD8+ TCM; p = 0.01). Regarding immune checkpoint molecules on CD4+ and CD8+ T cells, PD-1 expression on both T cells was markedly decreased regardless of treatment responses (Fig. 2C, CD4+ T cells in disease-controlled patients; p = 0.001, CD4+ T cells in disease-progressed patients; p = 0.0002, CD8+ T cells in disease-controlled patients; p = 0.001, CD8+ T cells in disease-progressed patients; p = 0.0005).
Fig. 2.
Alterations in the proportion of T cell subsets, activated T cells, and immune checkpoint molecule-expressing T cells in the peripheral blood of R/M HNSCC patients treated with nivolumab. The peripheral blood samples collected at two time points, pre-treatment and post-treatment, from 24 R/M HNSCC patients treated with nivolumab were analyzed and compared. a The proportion of T cell subsets for each CD4+ and CD8+ T cells. b The proportion of CD38+ and Ki-67+ cells for each CD4+ and CD8+ T cells. c The proportion of the immune checkpoint molecules, CTLA-4, PD-1, Lag-3, and Tim-3 expressing T cells, for each CD4+ and CD8+ T cells
Mass cytometry analysis
For a further detailed analysis of the expression patterns of immune checkpoint molecules and activation markers on T cell subsets, the blood samples from eight patients, four patients with PR/SD and four patients with PD, were analyzed using mass cytometry. Figure 3a shows the similarities before and after nivolumab treatment using the MDS plot. The distance between samples was calculated based on the expression of the molecules tested. T cells were clustered into eight T cell subsets according to the expression of CD4, CD8, CD45RO, CD45RA, CD27, and CCR7 (Supplementary Fig. 3a). Visualization was then carried out using UMAP (Fig. 3b). Representative marker expression in T cells is shown in Fig. 3c. The log2-fold change in each cluster between before and after nivolumab treatment was examined (Supplementary Fig. 3b). Nivolumab treatment resulted in an increase in the proportion of CD8+ T cells, CD4+ TEMRA, and CD8+ TEMRA subsets in disease-controlled patients (Supplementary Fig. 3c and d). Moreover, the expression levels of immune checkpoint molecules on T cells and their changes of each T cell subset before and after nivolumab treatment were examined by treatment efficacy, respectively (Fig. 3d–h). As shown in Fig. 3d and e, PD-1 expression in both CD4+ and CD8+ T cells was decreased after nivolumab treatment regardless of treatment response, and Tim-3 expression in CD8+ T cells after nivolumab treatment was higher in patients with disease progression. These results were consistent with those of flow cytometry analysis. The change in OX-40 expression in both CD4+ and CD8+ T cells was decreased in patients with disease progression, while that of CTLA-4 expression in CD4+ T cells was decreased in disease-controlled patients (Fig. 3g and h).
Fig. 3.
Mass cytometry analysis of R/M HNSCC patients treated with nivolumab. a Multi-dimensional scaffold plot representing 8 patients at two time points: before treatment initiation and after 4 infusions of nivolumab. b Cluster identification visualized using UMAP. c Expression level of the four representing lineage markers, CD4, CD8, CCR7, and CD45RA. d–h Expression level of immune checkpoint molecules on T cells. UMAPs show the normalized expression levels of checkpoint molecules. Bar graphs demonstrate the comparison of the expression levels between before and after nivolumab treatment across treatment efficacy. Heatmaps represent the change in the expression levels after nivolumab treatment compared to baseline (pre-treatment) in each T cell subset across treatment efficacy. The values in the heatmap were calculated by subtracting baseline. UMAP, uniform manifold approximation and projection. The asterisk (*) indicates p value less than 0.05
TAA-specific T cell responses and clinical responses to nivolumab
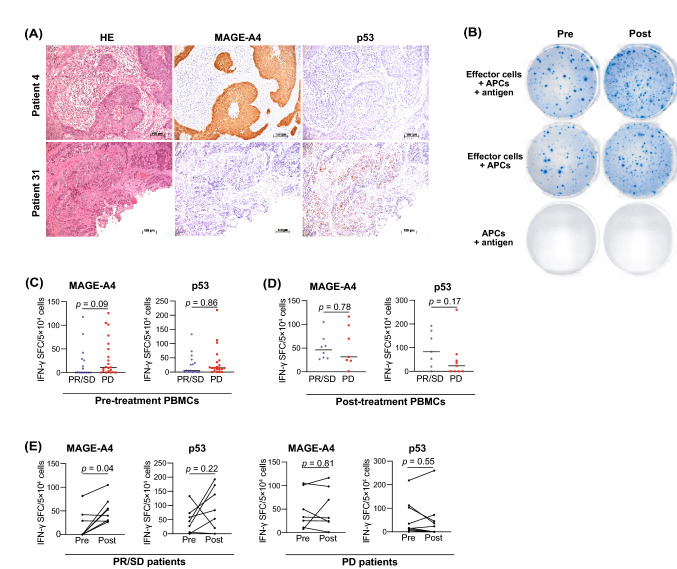
Flow cytometry and mass cytometry analysis revealed that nivolumab treatment resulted in the activation of effector T cells. We investigated the frequency of TAA-specific T cells before and after nivolumab treatment. For this purpose, two TAAs preferentially expressed in HNSCC, MAGE-A4, and p53, were selected. We first used IHC to investigate the expression of MAGE-A4 and p53 in tumor tissues obtained from 36 patients treated with nivolumab. Representative immunohistochemical staining for MAGE-A4 and p53 is shown in Fig. 4a. MAGE-A4 and p53 expression was detected in 21 (58.3%) and 24 (66.7%) patients, respectively (Table 1). Unfortunately, there was no correlation between TAA expression and treatment response (Supplementary Table 3). We proceeded to investigate the frequency of TAA-specific T cells in the PBMCs of TAA-positive patients before and after nivolumab treatment using the ELISPOT assay (Fig. 4b). Although the frequency of MAGE-A4-and p53 specific T cells was not significantly different between disease-controlled and disease-progressed patients before or after nivolumab treatment (Fig. 4c and d), the frequency of MAGE-A4-specific T cells was significantly increased in disease-controlled patients but not in disease-progressed patients after nivolumab treatment (Fig. 4e, disease-controlled patients; p = 0.04, disease-progressed patients; p = 0.81). There was no significant difference in the frequency of p53-specific T cells (Fig. 4e, disease-controlled patients; p = 0.22, disease-progressed patients; p = 0.55); however, 4 (57.1%) of the 7 patients with PR/SD showed a significant increase in the frequency of p53-specific T cells after nivolumab treatment.
Fig. 4.
TAA-specific response and clinical responses to nivolumab. The expression level of two TAAs, MAGE-A4 and p53, in tumor tissues was investigated, and the frequency of TAA-specific T cells in the PBMCs of TAA-positive patients collected before and after nivolumab treatment was measured. a Representative hematoxylin and eosin staining and immunohistochemistry for MAGE-A4 and p53. Patient 4: MAGE-A4-positive and p53-negative case, patient 31: MAGE-A4-negative and p53-positive case (×100 magnification). b Representative well imaging of ELISPOT assays to detect p53-specific T cell responses before and after treatment. c Comparison of the frequency of TAA-specific T cells between patients with disease progression and disease progression before treatment. d Comparison of the frequency of TAA-specific T cells between patients with disease control and disease progression post-treatment. e Alterations in the frequencies of TAA-specific T cells pre- and post-treatment based on treatment efficacy
Discussion
Here, we analyzed peripheral blood samples collected from R/M HNSCC patients treated with nivolumab. Unfortunately, the parameters investigated in this study, including the proportion of T cell subsets, activated T cells, and immune checkpoint molecule-expressing T cells, and the frequency of TAA-specific T cells, are unlikely to be predictive markers for treatment efficacy. Nonetheless, we found several novel points to understand the immunokinetics caused by ICI treatment. As expected, in disease-controlled patients, the proportion of TEMRA cells, particularly CD4+ T cells, increased after nivolumab treatment. This finding was confirmed by mass cytometry analysis. Moreover, the proportion of activated (CD38+) CD4+ and CD8+ TEMRA cells was significantly increased in the disease-controlled patients. Both CD4+ and CD8+ TEMRA cells are known to be more differentiated in terms of effector function, display potent cytotoxicity, and produce IFN-γ upon stimulation [23–26]. In non-small cell lung cancer patients, Kunert et al. [27] demonstrated that CD8+ T cell populations in partial response patients showed enhanced frequencies of TEMRA cells. Regarding CD4+ T cells, Pirozyan et al. [28] revealed increased IFN-γ production by CD4+ TEMRA cells in baseline samples from the responder group compared with the acquired resistance group. These findings suggest that the immune function of TEMRA cells can be used to predict responders to ICIs. Although our study failed to show any correlation between the proportion of CD4+ and CD8+ TEMRA cells or activated CD4+ and CD8+ TEMRA in baseline samples and treatment efficacy, at least in disease-controlled patients, activated CD4+ and CD8+ TEMRA would play pivotal roles in exerting antitumor effects.
Several studies on T cell activation have measured the expression of Ki-67, a nuclear protein associated with cellular proliferation [15, 29, 30]. In the present study, the proportion of Ki-67+ CD4+ and CD8+ T cells failed to demonstrate any significance in clinical outcomes in patients treated with nivolumab. However, another activation marker, CD38-expressing T cells, particularly CD8+ T cells, showed remarkable changes during nivolumab treatment in disease-controlled patients. Wu et al. reported that CD38+ CD8+ T cells expressed higher levels of cytotoxic molecules, cytokines, and PD-1 than CD38-CD8+ T cells in lung cancer [31]. Moreover, CD38+ CD8+ T cells isolated from tumors can be reinvigorated by anti-PD-1 in vitro. Clarke et al. [32] also carried out single-cell transcriptome analysis of cytotoxic T lymphocytes from anti-PD-1 responders, which revealed a significant enrichment of markers linked to activation (CD38) in post-treatment samples compared with pre-treatment samples. Thus, CD38-expressing CD8+ T cells in peripheral blood as well as tumors of patients treated with nivolumab would be reinvigorated and would function as antitumor effector cells.
Among the immune checkpoints tested, PD-1 expression on T cells was markedly decreased in all T cell subsets, regardless of treatment efficacy. As the anti-PD-1 antibody (MIH4) used for flow cytometry binds to an epitope that is distinct from the epitope bound by nivolumab, the decrease in PD-1 expression on T cells after nivolumab treatment could not be explained by PD-1 epitope saturation by nivolumab. Several studies have demonstrated that PD-1 expression on T cell subpopulations was decreased by in vitro PD-1 blockade with nivolumab using flow cytometry and RT-PCR [33, 34]. Likewise, our results revealed that nivolumab treatment reduced PD-1 expression in all T cells in vivo. Although the mechanisms underlying the downregulation of PD-1 on T cells remain largely unclear, one possibility is that PD-1-expressing T cells reinvigorated by nivolumab exhibit their effector function and finally undergo apoptosis. In addition, non-exhausted T cells that have not yet expressed PD-1 may emerge. As this downregulation was observed in both disease-controlled and disease-progressed patients, this phenomenon could not be limited to antitumor T cell populations. Interestingly, Lag-3 and Tim-3 expression on CD8+ T cells in patients with disease progression was significantly higher than that in disease-controlled patients after treatment. The findings of mass cytometry analysis of Tim-3 on CD8+ T cells also support this result. Koyama et al. showed that Tim-3 is upregulated owing to adaptive resistance to anti-PD-1therapy in both mouse models and patients [35]. In HNSCC, Shayan et al. revealed that the PD-1 blockade of tumor-infiltrating lymphocytes (TILs) led to further Tim-3 upregulation in a PI3K/Akt-dependent manner. In a murine HNC tumor model that is partially responsive to anti-PD-1 therapy, Tim-3 was upregulated in TILs from persistently growing tumors [36]. Our results showed similar Tim-3 upregulation in PBMCs at treatment failure. Currently, clinical trials of anti-Tim-3 antibody combined with anti-PD-1 antibody have already reported that this combination treatment was well tolerated and showed preliminary signs of antitumor activity [37]. Additionally, the altered expression of OX40 on CD8+ T cells and CTLA-4 on CD4+ T cells showed different behaviors between the disease-controlled and progressed patients. Thus, the expression of immune checkpoint molecules, besides PD-1, on T cells, particularly CD8+ T cells, may be a key mechanism of resistance to ICI treatments.
Although CD4+ or CD8+ TEMRA T cells were found to proliferate and were reinvigorated by nivolumab treatment, it is unknown whether effector T cells can recognize and kill tumor cells. To address this issue, we investigated the frequency of TAA-specific T cell responses in patients treated with nivolumab. The frequency of MAGE-A4- or p53-specific T cells before and after nivolumab treatment was not related to treatment efficacy. These findings suggest that the presence or frequency of MAGE-A4- and p53-specific T cells would have no direct effect on treatment efficacy. However, in disease-controlled but not disease-progressed patients, the frequency of MAGE-A4-specific T cells significantly increased after nivolumab treatment. Such findings suggest that some populations of effector T cells are activated by nivolumab and lyse tumor cells, resulting in the release of tumor antigens and subsequent induction of effector T cells, ultimately maintaining the cancer immunity cycle in disease-controlled patients. To date, only few studies have analyzed TAA-specific T cell responses in patients responding to nivolumab. Merhi et al. revealed that the NY-ESO-1-specific T cell response in a patient with recurrent HNSCC increased after nivolumab treatment [38]. In contrast, Veatch et al. reported that CD4+ and CD8+ T cell clones specific for lineage-specific antigens and cancer testis antigens did not expand in frequency in the blood during successful treatment in a patient with melanoma [39]. Thus, the kinetics of TAA-specific T cell response may differ depending on the type of cancer, the type of TAAs, expression level of TAAs, and immune status. Although accumulating evidence indicates that the treatment efficacy of ICIs is likely attributable to neoantigen-specific T cells [40–42], the immunokinetics of TAA-specific T cells may also indirectly reflect treatment efficacy.
In conclusion, in R/M HNSCC patients treated with nivolumab, circulating T cells showed dynamic alterations depending on treatment efficacy. Although further studies with a larger number of HNSCC patients treated with nivolumab will be necessary, an analysis of the immunokinetics of circulating T cells would provide new insights into rational therapeutic strategies in cancer immunotherapy for HNSCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- CyTOF
Cytometry by time-of-flight
- DAB
3,3’-Diaminobenzidine
- ELISPOT
Enzyme-linked immunosorbent spot
- FCS
Fetal calf serum
- Foxp3
Forkhead box P3
- HNSCC
Head and neck squamous cell carcinoma
- IFN
Interferon
- IHC
Immunohistochemistry
- ICI
Immune checkpoint inhibitor
- IL
Interleukin
- Lag-3
Lymphocyte activation gene 3
- MAGE-A4
Melanoma-associated antigen-A4
- MDS
Multi-dimensional scaling
- PBMC
Peripheral blood mononuclear cell
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-1 ligand 1
- PHA
Phytohemagglutinin
- R/M
Recurrent/metastatic
- SFC
Spot-forming-cells
- TAA
Tumor-associated antigen
- TCM
Central memory T cells
- TEM
Effector memory T cells
- TEMRA
Terminally differentiated effector memory T cells
- Tim-3
T cell immunoglobulin mucin-3
- TN
Naïve T cells
- UMAP
Uniform manifold approximation and projection
Author contributions
Hideyuki T. and KC were involved in conception and design. Hiroe T., Hideyuki T., KY, KM, YN, MU, MS, SI, IM, TM, KT and HS were involved in sample preparation and data acquisition. Hiroe T., Hideyuki T., TO, ST and KC were involved in analysis and interpretation of data. Hiroe T. and KC were involved in writing of the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research (Grant No. 20K18243 to Hideyuki T., No. 20K09747 to M.S., No. 19K18794 to S.I., No 19K18758 to I.M., and No. 20H03834 to K.C.) from the Ministry of Education, Culture, Sports, Science and Technology; a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Research Project for Sericultural Revolution), Japan; and Financial Support for the Promotion of University-Industry Collaborative Research Based on Regulatory Sciences at Gunma University.
Availability of data and material
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict interest.
Ethical approval
This study was approved by the Ethical Committee of Gunma University Hospital (HS2017-152).
Informed consent
Written informed consent was obtained from each patient.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hiroe Tada and Hideyuki Takahashi contributed equally to this study.
References
- 1.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 7.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol. 2019;99:104460. doi: 10.1016/j.oraloncology.2019.104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. doi: 10.1186/s40364-020-00209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otoshi T, Nagano T, Tachihara M, Nishimura Y. Possible biomarkers for cancer immunotherapy. Cancers (Basel) 2019;11:935. doi: 10.3390/cancers11070935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Nixon AB, Schalper KA, Jacobs I, Potluri S, Wang IM, Fleener C. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunother Cancer. 2019;7:325. doi: 10.1186/s40425-019-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez C, Arasanz H, Chocarro L, Bocanegra A, Zuazo M, Fernandez-Hinojal G, et al. Systemic blood immune cell populations as biomarkers for the outcome of immune checkpoint inhibitor therapies. Int J Mol Sci. 2020;21:2411. doi: 10.3390/ijms21072411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valpione S, Galvani E, Tweedy J, Mundra PA, Banyard A, Middlehurst P, et al. Immune-awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nat Cancer. 2020;1:210–221. doi: 10.1038/s43018-019-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol. 2018;9:1613. doi: 10.3389/fimmu.2018.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad R, Concha-Benavente F, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, et al. Nivolumab treatment beyond RECIST-defined progression in recurrent or metastatic squamous cell carcinoma of the head and neck in CheckMate 141: a subgroup analysis of a randomized phase 3 clinical trial. Cancer. 2019;125:3208–3218. doi: 10.1002/cncr.32190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Witzleben A, Wang C, Laban S, Savelyeva N, Ottensmeier CH. HNSCC: Tumour antigens and their targeting by immunotherapy. Cells. 2020;9:2103. doi: 10.3390/cells9092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watling DL, Gown AM, Coltrera MD. Overexpression of p53 in head and neck cancer. Head Neck. 1992;14:437–444. doi: 10.1002/hed.2880140603. [DOI] [PubMed] [Google Scholar]
- 20.Nowicka M, Krieg C, Crowell HL, Weber LM, Hartmann FJ, Guglietta S, et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res. 2017;6:748. doi: 10.12688/f1000research.11622.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada K, Masuda K, Ida S, Tada H, Bando M, Abe K, et al. In vitro assessment of antitumor immune responses using tumor antigen proteins produced by transgenic silkworms. J Mater Sci Mater Med. 2021;32:58. doi: 10.1007/s10856-021-06526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motokawa Y, Kokubo M, Kuwabara N, Tatematsu KI, Sezutsu H, Takahashi H, et al. Melanoma antigen family A4 protein produced by transgenic silkworms induces antitumor immune responses. Exp Ther Med. 2018;15:2512–2518. doi: 10.3892/etm.2018.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 25.Reading JL, Gálvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol Rev. 2018;283:194–212. doi: 10.1111/imr.12657. [DOI] [PubMed] [Google Scholar]
- 26.Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun. 2017;8:1473. doi: 10.1038/s41467-017-01728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunert A, Basak EA, Hurkmans DP, Balcioglu HE, Klaver Y, van Brakel M, et al. CD45RA+CCR7- CD8 T cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of NSCLC patients responding to nivolumab. J Immunother Cancer. 2019;7:149. doi: 10.1186/s40425-019-0608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirozyan MR, McGuire HM, Emran AA, Tseng HY, Tiffen JC, Lee JH, et al. Pretreatment innate cell populations and CD4 T cells in blood are associated with response to immune checkpoint blockade in melanoma patients. Front Immunol. 2020;11:372. doi: 10.3389/fimmu.2020.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osa A, Uenami T, Koyama S, Fujimoto K, Okuzaki D, Takimoto T, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3:e59125. doi: 10.1172/jci.insight.59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee SH, et al. The first-week proliferative response of peripheral blood PD-1+CD8+ T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25:2144–2154. doi: 10.1158/1078-0432.CCR-18-1449. [DOI] [PubMed] [Google Scholar]
- 31.Wu P, Zhao L, Chen Y, Xin Z, Lin M, Hao Z, et al. CD38 identifies pre-activated CD8+ T cells which can be reinvigorated by anti-PD-1 blockade in human lung cancer. Cancer Immunol Immunother. 2021 doi: 10.1007/s00262-021-02949-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O, et al. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med. 2019;216:2128–2149. doi: 10.1084/jem.20190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puntigam LK, Jeske SS, Götz M, Greiner J, Laban S, Theodoraki MN, et al. Immune checkpoint expression on immune cells of HNSCC patients and modulation by chemo- and immunotherapy. Int J Mol Sci. 2020;21:5181. doi: 10.3390/ijms21155181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding G, Shen T, Yan C, Zhang M, Wu Z, Cao L. IFN-γ down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC Cancer. 2019;19:1053. doi: 10.1186/s12885-019-6145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2017;6:e1261779. doi: 10.1080/2162402X.2016.1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM, et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin Cancer Res. 2021;27:3620–3629. doi: 10.1158/1078-0432.CCR-20-4746. [DOI] [PubMed] [Google Scholar]
- 38.Merhi M, Raza A, Inchakalody VP, Nashwan AJJ, Allahverdi N, Krishnankutty R, et al. Squamous cell carcinomas of the head and neck cancer response to programmed cell death Protein-1 targeting and differential expression of immunological markers: a case report. Front Immunol. 2018;9:1769. doi: 10.3389/fimmu.2018.01769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veatch JR, Singhi N, Jesernig B, Paulson KG, Zalevsky J, Iaccucci E, et al. Mobilization of pre-existing polyclonal T cells specific to neoantigens but not self-antigens during treatment of a patient with melanoma with bempegaldesleukin and nivolumab. J Immunother Cancer. 2020;8:e001591. doi: 10.1136/jitc-2020-001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Łuksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017;551:517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.