Abstract
Purpose
To determine performances of 2-deoxy-2-(18F)fluoro-d-glucose (18F-FDG) positron emission tomography (PET) to detect the development of permanent thyroid dysfunction (PTD), and to evaluate the prognostic value of early increased thyroid uptake in stage IV melanoma patients treated with anti-programmed death 1 (anti-PD-1) antibodies.
Methods
Twenty-nine patients were retrospectively enrolled. PTD was defined as symptomatic thyroid disorder requiring long-term specific treatment. On the first PET performed during follow-up, maximal standardized uptake value of the thyroid (SUVmax-Th) and SUVmax-Th/SUVmax-blood-pool ratio (Th/B) were measured. Areas under ROC curves (AUC) of these parameters for the diagnostic of PTD were compared. Cutoff values were defined to maximize the Youden’s index. Survival analyses were performed according to the Kaplan–Meier method and compared using the log-rank method between patients with and without enhanced thyroid uptake according to cutoff values defined with the Hothorn and Lausen method.
Results
Four patients presented PTD. Median SUVmax-Th and Th/B were, respectively, 2.11 and 1.00. The median follow-up period was 21.7 months. AUC were 1.0 (CI95% 0.88–1.0) for both parameters. Optimal cutoff values were, respectively, SUVmax-Th > 4.1 and Th/B > 2.0, both conferring sensitivities of 100% (CI95% 40–100%) and specificities of 100% (CI95% 86–100%). The median progression-free survival and overall survival were 11.3 months and 33.5 months, respectively. Using optimized cutoffs, there was no statistically significant difference of survival.
Conclusion
SUVmax-Th > 4.1 and Th/B > 2.0 provided perfect diagnostic performances to detect patients that developed PTD. No significant survival difference was found between patients with and without increased thyroid uptake.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02712-7) contains supplementary material, which is available to authorized users.
Keywords: 18F-FDG PET/CT, Metastatic melanoma, Anti-PD-1 antibodies, Thyroid, Immune-related adverse events, Immune checkpoint inhibitors
Introduction
Immune checkpoint inhibitors, including anti-programmed death 1 (anti-PD-1) inhibitors such as pembrolizumab or nivolumab can restore antitumor immunity by enhancing antitumor T-cell lymphocytes response. Such therapies have significatively improved the prognosis of patients with advanced-stage melanoma [1–3]. However, the development of these monoclonal antibodies has led to the emergence of immune-related adverse events (irAEs) which have been described in almost all organs [4]. Thyroid dysfunctions are one of the most frequent side effects with an incidence of 10–20% in patients treated with PD-1 blockade [5–7]. Inflammatory thyroiditis with transient thyrotoxicosis often followed by hypothyroidism is the most frequent patterns of thyroid disorders [8–10].
2-Deoxy-2-(18F)fluoro-d-glucose (18F-FDG) positron emission tomography associated with computed tomography (PET/CT) is used in clinical practice to assess the efficacy of melanoma therapies [11]. In patients treated- with immunotherapy, 18F-FDG PET/CT can also detect several immune-mediated side effects [12]. Indeed, immune inflammatory reactions observed in irAEs can result in increased 18F-FDG uptake in the relevant organs. Some authors reported a diffusely increased uptake in the thyroid gland related to inflammatory thyroiditis in patients treated with anti-PD-1 for melanoma or lung cancer, and suggested a good predictive positive value of 18F-FDG PET/CT [5, 13, 14]. However, parameters and threshold values used to define increased thyroid uptake are highly heterogeneous.
The association between irAES and higher response rate to immunotherapy have been described [15–17] but remains discussed. The occurrence of irAEs may reflect the activation of immune system necessary for the antitumor response. Increased 18F-FDG uptake in the thyroid gland could stand for a surrogate of immunotherapy response [18]. In a previously published study of Kotwal et al. [19], thyroid uptake was associated with the occurrence of thyroid dysfunctions in patients treated with PD-L1 inhibitors for various cancer. In the present study, we propose to investigate the association of an increased thyroid uptake with the occurrence of permanent thyroid dysfunctions (PTD) and clinical outcomes in another kind of population with melanoma patients treated with PD-1 inhibitors.
This retrospective study aims at achieving two main objectives: (1) to determine performances of 18F-FDG PET/CT to identify patients that develop PTD, and (2) to evaluate the prognostic value of increased thyroid 18F-FDG uptake for progression-free survival (PFS) and overall survival (OS) of patients with stage IV melanoma treated with anti-PD-1 antibodies.
Patients and methods
Patients
Between January 2015 and December 2017, patients with stage IV melanoma who started anti-PD-1 antibodies (pembrolizumab or nivolumab) alone or in association with ipilimumab in our center, and performed 18F-FDG PET/CT at baseline (in the three months before the beginning of immune checkpoint inhibitors) and at least one 18F-FDG PET/CT on treatment were retrospectively enrolled. For all included patients, clinical and biological data were collected from electronic medical records, and no critical information was missing. A simplified declaration to the French data protection authority (CNIL) under the number 208821 was performed for this study.
FDG PET/CT
Patients were fasting for 6 h before 18F-FDG injection. Image acquisitions were performed 1 h after injection of 3Mbq/kg 18F-FDG, on a Biograph™ mCT (Siemens Healthcare, Erlangen, Germany) or Discovery ST (GE Healthcare, Chicago, Illinois, USA) integrated PET/CT system. PET images were reconstructed with attenuation correction based on coregistered CT, in 3-dimentional mode from the vertex to the pelvis or to the toes, using iterative algorithms. In all 18F-FDG PET/CT from baseline to latest follow-up, a senior nuclear medicine physician measured two quantitative parameters: SUVmax of the thyroid gland (SUVmax-Th) and SUVmax thyroid/SUVmax blood pool ratio (Th/B). Imaging follow-up during and after anti-PD-1 was performed using 18F-FDG PET/CT. All images were carefully reread and rated according to PET Response Evaluation Criteria for Immunotherapy (PERCIMT) [20]. Patients who presented partial or complete metabolic response as the best overall response during anti-PD1 were classified as responders.
Thyroid dysfunctions and survival analysis
TSH, free T3 and free T4 values were collected during the treatment and until the date of latest follow-up transitory thyroid dysfunction was defined as asymptomatic thyroid biological disorders without specific treatment required and included hyperthyroidisms and hypothyroidisms. According to guidelines on iatrogenic thyroid disorders under immune checkpoint inhibitors proposed by Illouz et al. l-thyroxin substitution was indicated in clinical hypothyroidism with TSH elevation on two assays and low fT4 or TSH > 10 mIU/L [21]. Thus, in this current study, PTD was defined as symptomatic thyroid disorders and included hyperthyroidisms permanently treated with antithyroid drugs or hypothyroidisms permanently treated with levothyroxine substitution. PFS was defined as the time from the first day of treatment with PD-1 inhibitors to the day of progressive disease according to imaging follow-up.
Statistical analysis
Statistical analyses were performed with MedCalc® 12.5.0.0 (MedCalc Software bvba, Ostend, Belgium). For descriptive statistics, median [interquartile range (IQR) 1st quartile to 3rd quartile] was used. Receiver operating characteristics (ROC) curves of ability for both PET parameters on the first PET/CT during follow-up (SUVmax-Th and Th/B) to, respectively, detect patients who will develop PTD. These 18F-FDG PET/CT parameters were, respectively, dichotomized based on thresholds providing the best Youden’s indexes (sensitivity + specificity − 1) [22, 23]. In the second part of this study, we dichotomized SUVmax-Th and Th/B to fit with PFS and OS, respectively, according to the Hothorn and Lausen method using the “maxstat” R package [24]. For each feature, the group with high thyroid uptake and the one with low thyroid uptake were compared regarding PFS and OS calculated using the Kaplan–Meier method, with censoring at the time of the last follow-up. PFS and OS were, respectively, compared between both groups using nonparametric log-rank tests. Finally, areas under the ROC curves representing diagnostic performances of SUVmax-Th and Th/B, respectively, to detect responders were calculated. A p value smaller than 0.05 was considered to be statistically significant.
Results
Patients characteristics
Twenty-nine patients were analyzed, aged of 68 years-old [IQR 53–76] (Table 1). The median duration of immunotherapy was 10.1 months [IQR 3.4–16.8], and median follow-up period was 21.7 months [IQR 11.6–34.2]. The first 18F-FDG PET/CT on anti-PD-1 antibodies was performed after a median of five [IQR 4–6] cycles of treatment. Four patients (14%) presented permanent hypothyroidism requiring levothyroxine substitution, including one patient (patient 23) who had been diagnosed with Grave’s disease prior to the start of anti-PD-1 antibodies. Eight patients (28%) had transitory thyroid dysfunction. None of the patients had permanent hyperthyroidism. Death occurred in 14 (48%) patients and 22 (76%) had progressive disease during the follow-up period.
Table 1.
Patient characteristics at day 1 of anti-PD-1 and incidence of thyroid dysfunctions
| Patients | n = 29 (%) |
|---|---|
| Age, median (years old) | 68 [IQR 53–76] |
| Sex | |
| Males | 14 (48%) |
| Females | 15 (52%) |
| Melanoma metastatic stage (AJCC 8th edition) at day 1 | |
| m1a | 10 (35%) |
| m1b | 5 (17%) |
| m1c | 10 (35%) |
| m1d | 4 (13%) |
| Number of previous metastatic treatment lines | |
| None | 18 (62%) |
| One | 7 (24%) |
| Two | 3 (10%) |
| Three | 1 (4%) |
| Including immune checkpoint inhibitors (ipilimumab) | 6 (21%) |
| Current treatment | |
| Pembrolizumab alone | 18 (62%) |
| Nivolumab alone | 10 (35%) |
| Nivolumab + Ipilimumab | 1 (3%) |
| Thyroid dysfunctions | |
| No dysfunction | 17 (58%) |
| Transitory thyroid dysfunctions | 8 (28%) |
| Permanent thyroid dysfunctions | 4 (14%) |
AJCC American joint committee of cancer
Diagnostic performances for PTD
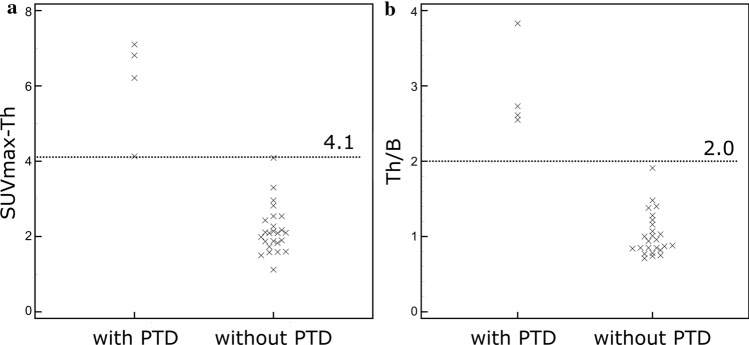
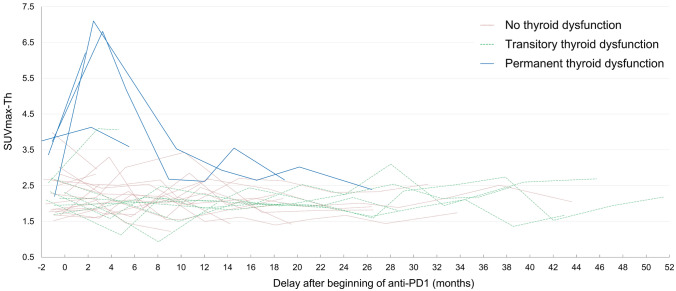
Median SUVmax-Th and Th/B were, respectively, 2.11 [IQR 1.87–2.82] and 1.00 [IQR 0.85–1.38] (supplementary material). AUC were 1.0 (CI95% 0.88–1.00) for both studied parameters. Cutoff values maximizing Youden’s index to detect PTD were, respectively, SUVmax-Th > 4.1, Th/B > 2.0 (Fig. 1), providing a sensitivity of 100% (CI95% 40–100%), a specificity of 100% (CI95% 86–100%), a positive predictive value of 100% (CI95% 40–100%) and a negative predictive value of 100% (CI95% 86–100%). All four patients who developed permanent hypothyroidism presented increased thyroid uptake (based on both studied parameters) on the first PET/CT on treatment, that did not last more than 6 months after the beginning of treatment for three out of four patients (Figs. 2, 3). The fourth patient had only one PET/CT on treatment. Interestingly, patient 23 who was previously diagnosed with Grave’s disease already had increased Th/B ratio (2.5) at baseline 18F-FDG PET/CT. Neither patients with transitory thyroid dysfunction nor those without thyroid dysfunction presented increased thyroid uptake. The four patients with increases thyroid uptake and PTD did not present any other 18F-FDG uptake that may be related to irAEs.
Fig. 1.
Distribution of a maximum standardized uptake value in the thyroid (SUVmax-Th) and b SUVmax-thyroid/SUVmax-blood-pool ratio (Th/B) for patients with and without permanent thyroid dysfunction (PTD) developed during follow-up. Retrospectively defined optimum threshold values are presented with dotted lines
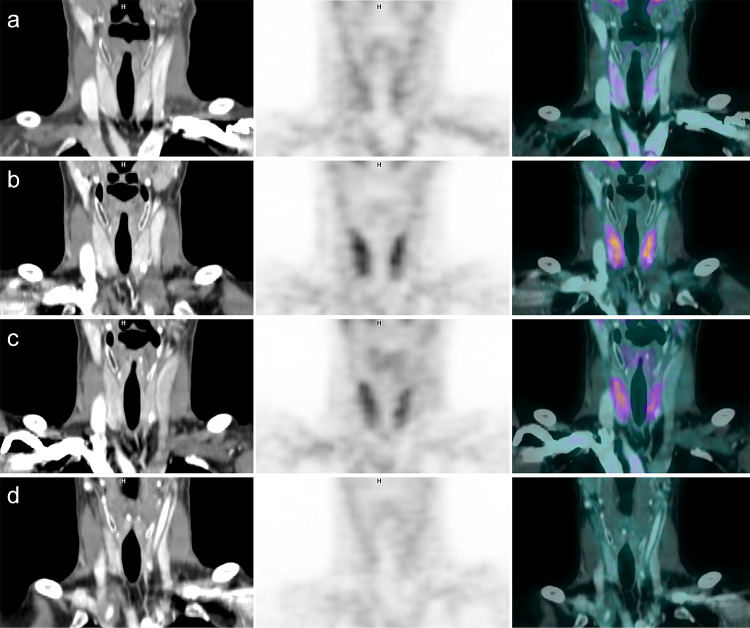
Fig. 2.
18F-FDG PET/CT images of patient nine who developed a permanent hypothyroidism. Images a were performed at baseline. Images b, c were, respectively, obtained 3 and 5 months after the beginning of nivolumab associated with ipilimumab, and revealed a diffusely increased thyroid uptake. Three months after the end of immune checkpoint inhibitors, images d showed a complete regression of the previously increased thyroid uptake and a significant decrease of thyroid volume on CT
Fig. 3.
Evolution of increased 18F-FDG thyroid uptake based on maximum standardized uptake value in the thyroid (SUVmax-Th) after the beginning of anti-PD-1 in all patients. All four patients who developed permanent hypothyroidism had an increased 18F-FDG thyroid uptake at the first restaging PET. Increased 18F-FDG thyroid uptake regressed in less than six months three out of four patients, and the fourth patient had only one PET after the onset of anti-PD-1
Survivals and response rates
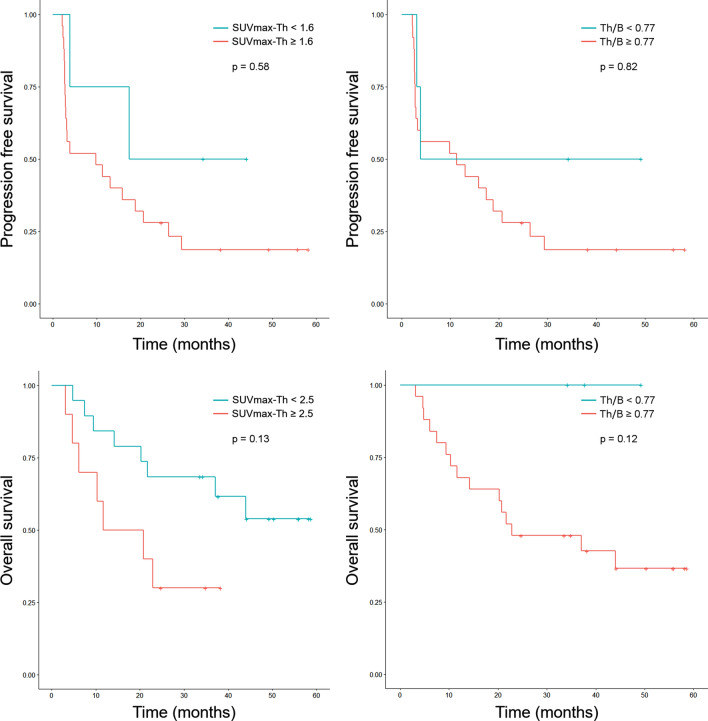
The median PFS and OS were 11.3 months [IQR 2.81–27.1] and 33.5 months [IQR 11.3–44.1], respectively. The best cutoff values to dichotomize patients based on thyroid uptake were SUVmax-Th = 1.6 and Th/B = 0.77 regarding PFS, and SUVmax-Th = 2.5 and Th/B = 0.77 regarding OS (Fig. 4). Based on Kaplan–Meier survival curves, median PFS was 9.8 months (CI95% 3.0–26.4) for the group of patients with SUVmax-Th ≥ 1.6 and 17.4 months (CI95% 3.9 to “not reached”) for those with SUVmax-Th < 1.6 (p = 0.58), and was 3.9 months (CI95% 3.2 to “not reached”) for the group with Th/B ≥ 0.77 and 11.3 months (CI95% 3.0–26.4) for those with SUVmax-Th < 0.77 (p = 0.82). Median OS was 20.7 months for the group of patients with SUVmax-Th ≥ 2.5 and not reached for those with SUVmax-Th < 2.5 (p = 0.13), and was 22.9 month for the group with Th/B Th ≥ 0.77 and not reached for those with Th/B < 0.77 (p = 0.12). Regarding tumor response, AUC were 0.70 (CI95% 0.50–0.85) for SUVmax-Th and 0.50 (CI95% 0.31–0.69) for Th/B, respectively, to detect patients classified as “responders”. Patients with lower SUVmax-Th tended to be more classified as “responders”.
Fig. 4.
Kaplan–Meier curves of progression free survival and overall survival compared between groups of patients with “high thyroid uptake” and “low thyroid uptake” regarding maximum standardized uptake value in the thyroid (SUVmax-Th) and SUVmax-thyroid/SUVmax-blood-pool ratio (Th/B)
Discussion
The first goal of our study was to determine the optimal diagnostic performances of 18F-FDG PET/CT to identify the development of PTD. Thus, we retrospectively determined objective threshold values based on two widely used 18F-FDG PET parameters that provided perfect sensitivity and specificity for the 29 studied patients. These values were, respectively, SUVmax-Th > 4.1, Th/B > 2.0 (Fig. 1). The second objective was to evaluate the prognostic value of increased thyroid 18F-FDG uptake regarding PFS and OS. Our results did not reveal any statistically significant difference neither for PFS nor OS between two groups of patients with and without increased thyroid uptake after the onset of anti-PD-1 antibodies.
Early detection of irAEs, in particular by 18F-FDG PET/CT, is currently a hot topic in literature. Increased thyroid 18F-FDG uptake has previously been reported in patients treated with immune checkpoint inhibitors who presented thyroid disorders [5, 12–14, 18, 19, 25, 26] with good sensitivity and excellent specificity to detect patients that will develop PTD. However, in these studies 18F-FDG PET/CT interpretation criteria, diseases and treatment were highly heterogeneous (Table 2). To our knowledge, there is no published study specifically investigating the association between an objectively defined increased thyroid uptake and the occurrence of PTD induced by anti-PD-1 therapy specifically in advanced melanoma patients.
Table 2.
Performances of 18F-FDG PET/CT to predict permanent thyroid dysfunctions on anti-PD-1: data from the literature
| First author | Year | n (cancer type) | Immunotherapy (n) | Criteria on PET | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| De Fillette [5] | 2016 | 99 (stage III or IV melanomas) | pembrolizumab | visual/semiquantitative evaluation (intense diffuse 18F-FDG uptake; no quantitative threshold) | 7/7 (100%) | NA |
| Delivanis [25] | 2017 | 13 patients (Stage IV melanomas n = 11, lung cancer n = 1) | pembrolizumab | visual evaluation (intense diffuse 18F-FDG uptake) | 7/11 (64%) | NA |
| Eshghi [13] | 2018 | 18 (lung cancer) | nivolumab | visual evaluation (thyroid uptake higher than blood pool) | 4/6 (67%) | NA |
| Mekki [14] | 2018 | 53 (melanomas n = 32, lung cancers n = 18, others n = 3) | nivolumab (n = 27) or pembrolizumab (n = 26) | visual evaluation (intense diffuse 18F-FDG uptake) | 4/4 (100%) | NA |
| Yamaushi [26] | 2019 | 200 (lung cancers n = 118, melanomas n = 42, others n = 40) | nivolumab | visual evaluation (intense diffuse 18F-FDG uptake) | 7/15 (47%) | 92/96 (96%) |
| Kowtal [19] | 2020 | 91 various cancers | atezolizumab (n = 86) or avelumab (n = 5) | visual evaluation (intense diffuse 18F-FDG uptake) | 4/4 (100%) | 18/20 (90%) |
| Current study | 2020 | 29 (stage IV melanomas) | pembrolizumab (n = 18), nivolumab (n = 10) or nivolumab + ipilumumab (n = 1) |
SUVmax-Th > 4.1 and/or Th/B > 2.0 |
4/4 (100%) | 25/25 (100%) |
| Pooled | 37/51 (73%) | 135/141 (96%) |
NA not available, SUVmax-Th maximum standardized uptake value in the thyroid, Th/B SUVmax-thyroid/SUVmax-blood-pool ratio
PTD as an irAE seems to be due to an inflammatory process [5] similar to those described in Hashimoto’s thyroiditis in which thyroid 18F-FDG uptake could be due to lymphocyte infiltrate in the thyroid gland [27]. Nevertheless, precise mechanisms underlying thyroid irAEs remain unknown, and the role of thyroid autoantibodies remains unclear [25]. Osorio et al. [6] described the presence of antithyroid antibodies (antithyroglobulin or antimicrosomal antibodies) in the majority of patients who developed thyroid dysfunctions during pembrolizumab treatment. However, it is uncertain whether the presence of antithyroid antibodies is the cause or the consequence of a destructive thyroiditis [27]. Indeed, one hypothesis is that the occurrence of a destructive thyroiditis induced by anti-PD-1 antibodies, causes the release of thyroid antigens leading to secondary production of thyroid auto-antibodies [28].
An increased 18F-FDG thyroid uptake before the onset of immunotherapy seems to be associated with the occurrence of PTD and is sometimes associated with baseline elevated thyroid autoantibodies [26]. Some authors suggested that a previously existing latent thyroid autoimmunity in some patients could be unmasked by the start of anti-PD-1 antibodies [6]. In the study of Yamauchi et al. [26], increased 18F-FDG uptake in the thyroid gland before the start of immunotherapy is associated with high incidence of overt thyroid irAEs but not with transient thyroid irAEs. In our study, one patient (patient 23) diagnosed with Grave’s disease had an increased 18F-FDG thyroid uptake before the start of anti-PD-1. He later developed a permanent hypothyroidism. Thus, 18F-FDG PET/CT could be of interest to identify patients with preexisting auto-immunity who are most at risk to develop permanent hypothyroidism. Moreover, 18F-FDG PET/CT could help to identify patients who will develop a permanent hypothyroidism before the biological signs during anti-PD-1 treatment. In the study of Eshghi et al. [13], two out of 4 patients who had increased thyroid 18F-FDG uptake had normal TSH at time of follow-up 18F-FDG PET/CT. In our study, one patient had an increased thyroid uptake less than two months after the start of treatment with normal TSH values at this time but developed a subsequent permanent hypothyroidism. Thus, an increased thyroid uptake on 18F-FDG PET/CT with normal TSH values should prompt the clinician to monitor symptoms of hypothyroidism and TSH values more regularly.
The link between irAEs and response to immunotherapy remains controversial. Several authors reported a positive association between immune toxicities and immunotherapy response in patients treated with anti-Cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4) [29] or anti-PD-1 [30], especially in patients with induced-vitiligo [31–33]. However, other studies have not found any association between toxicity and response or survival [34–36]. Concerning thyroid dysfunctions, results of studies are also contradictory. Freeman-Keller et al. [37] found no improvement in survival in patients with endocrinopathies treated with nivolumab for a metastatic melanoma. In the study of Yamauchi et al. [26], patients with lung cancer treated with nivolumab who presented thyroid dysfunctions had a better median OS but this observation was not seen in patients with malignant melanoma. Similarly, in the study of Peiro et al. [38], improved OS was only seen in patients with lung cancer. More recently, in a prospective study, Basak et al. [39] showed that overt thyroid dysfunctions were associated with an improved OS and PFS in a cohort of patients with a majority of lung cancer treated with anti-PD-1. Increased response and survival in patients with irAEs could be explained by common mechanisms between immune antitumor response and autoimmunity. Nevertheless, those mechanisms appear to be complex and are not fully understood.
18F-FDG PET/CT can detect various immune-mediated side effects in patients treated with immune checkpoint inhibitors such as pancreatitis, hepatitis, arthritis, enterocolitis, hypophysitis, reactive lymphadenopathies (sarcoid-like syndrome), interstitial pneumonitis [14, 18, 40]. Several authors suggested that those immune-mediated side effects detected on 18F-FDG PET/CT could be associated with improved survival or response to anti-PD-1 or anti-CTLA-4 therapies. In their study, Sachpekidis et al. [17] reported that all ten metastatic melanoma patients with sarcoid-like lymphadenopathy achieved a disease control on ipilimumab. Likewise, in the study of Nobashi et al. [18], nine of eleven patients with immune side effects induced by anti-PD-1 or anti-CTLA-4 were in complete response at the last follow-up scan. In particular, all five patients with an increased 18F-FDG thyroid uptake at the first restaging scan had a complete response in 1 year. In contrast, in our study, there was no statistically significant difference neither for PFS nor OS between patients with and without increased thyroid uptake. Interestingly, patients with low SUVmax-Th and Th/B tended to have better PFS, OS and response rate than the others. These results go against our initial hypothesis. Indeed, we initially hypothesized that increased 18-FDG thyroid uptake associated with thyroid autoimmunity, might be indicative of tumor response. An association between favorable outcomes and thyroid dysfunctions has been reported in lung cancer [6, 16, 26, 38]. This correlation has not been evidenced yet in a population of melanoma patients [41]. In addition, some authors highlighted that the prognostic significance of irAEs may vary depending on the primitive tumor site and toxicity type. According to Freeman-Keller and al [37]. only cutaneous side effects but neither colitis, pneumonitis nor thyroid dysfunctions were associated with clinical benefit in melanoma patients on anti-PD-1. Common antigens between normal melanocytes and melanoma cells could explain the association between induced vitiligo and clinical benefit under anti-PD-1, only observed in melanoma patients [33]. On the other hand, the specific mechanism of thyroid dysfunctions induced by anti-PD-1 antibodies remains unclear [5, 19, 28].
This study presents some limitations. First, the small size of the single-center cohort and the low number of patients who developed PTD may have limited the statistical power of our results. Secondary, due to the retrospective design, some biological data such as TSH, T3, T4 and antithyroid antibodies were missing at some time points during follow-up. The retrospectively defined threshold values that we proposed to identify patients who developed PTD need to be confirmed in larger prospective studies.
In conclusion, objective threshold values of SUVmax-Th > 4.1 and Th/B > 2.0 provided perfect sensitivity, specificity, VPP and VPN to detect patient that developed of PTD as early as the first 18F-FDG PET/CT after the onset of anti-PD-1 antibodies in patients with stage IV melanoma. Thus, increased 18F-FDG uptake on thyroid gland during anti-PD-1 treatment should alert the clinician to closely monitor TSH values. Furthermore, such objective cutoff values may be useful to improve interstudy comparison of forthcoming research focusing on thyroid irAEs on 18F-FDG PET/CT. No statistically significant survival difference was found between patients with and without increased thyroid uptake.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material. Histogram of maximum standardized uptake value in the thyroid (SUVmax-Th) and SUVmax-thyroid/SUVmax-blood-pool ratio (Th/B) in patients who developed permanent thyroid dysfunction (PTD) or not. file1 (TIFF 13125 kb)
Abbreviations
- 18F-FDG
2-Deoxy-2-(18F)fluoro-d-glucose
- AJCC
American joint committee of cancer
- Anti-PD-1
Anti-programmed death 1 antibodies
- AUC
Areas under ROC curves
- CT
Computed tomography
- irAEs
Immune-related adverse events
- NA
Not available
- OS
Overall survival
- PET
Positron emission tomography
- PFS
Progression-free survival
- PTD
Permanent thyroid dysfunction
- ROC
Receiver operating characteristics
- SUVmax
Maximal standardized uptake value
- SUVmax-Th
Maximal standardized uptake value of the thyroid
- Th/B
Ratio SUVmax-Th/SUVmax-blood-pool
- TSH
Thyroid stimulating hormone
Author contributions
AF, AG and TLS designed the study. AF, AG, EJ and LB participated in the collection of data. AG performed the statistical analysis. AF and AG drafted the manuscript. XPN, LB, AD, AD, FL, JE and PJ provided their critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes.
Informed consent
Because of the retrospective nature of this study, consent was not obtained. A simplified declaration to the French data protection authority (CNIL) under the number 208821 was performed for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M et al. (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol [DOI] [PubMed]
- 5.De Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101:4431–4439. doi: 10.1210/jc.2016-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28:583–589. doi: 10.1093/annonc/mdw640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morganstein DL, Lai Z, Spain L, Diem S, Levine D, Mace C, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2017;86:614–620. doi: 10.1111/cen.13297. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 9.Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243–1251. doi: 10.1089/thy.2018.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R, et al. Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res. 2017;5:1133–1140. doi: 10.1158/2326-6066.CIR-17-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salaün P-Y, Abgral R, Malard O, Querellou-Lefranc S, Quere G, Wartski M, et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur J Nucl Med Mol Imaging. 2020;47:28–50. doi: 10.1007/s00259-019-04553-8. [DOI] [PubMed] [Google Scholar]
- 12.Wachsmann JW, Ganti R, Peng F. Immune-mediated disease in ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol. 2017;24:111–115. doi: 10.1016/j.acra.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Eshghi N, Garland LL, Nia E, Betancourt R, Krupinski E, Kuo PH. 18F-FDG PET/CT can predict development of thyroiditis due to immunotherapy for lung cancer. J Nucl Med Technol. 2018;46:260–264. doi: 10.2967/jnmt.117.204933. [DOI] [PubMed] [Google Scholar]
- 14.Mekki A, Dercle L, Lichtenstein P, Marabelle A, Michot J-M, Lambotte O, et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer. 2018;96:91–104. doi: 10.1016/j.ejca.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Bronstein Y, Ng CS, Hwu P, Hwu W-J. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti–CTLA-4 antibody therapy. Am J Roentgenol. 2011;197:W992–1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 16.Sachpekidis C, Kopp-Schneider A, Hakim-Meibodi L, Dimitrakopoulou-Strauss A, Hassel JC. 18F-FDG PET/CT longitudinal studies in patients with advanced metastatic melanoma for response evaluation of combination treatment with vemurafenib and ipilimumab. Melanoma Res. 2019;29:178–186. doi: 10.1097/CMR.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 17.Sachpekidis C, Larribère L, Kopp-Schneider A, Hassel JC, Dimitrakopoulou-Strauss A. Can benign lymphoid tissue changes in 18F-FDG PET/CT predict response to immunotherapy in metastatic melanoma? Cancer Immunol Immunother. 2019;68:297–303. doi: 10.1007/s00262-018-2279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobashi T, Baratto L, Reddy SA, Srinivas S, Toriihara A, Hatami N, et al. Predicting response to immunotherapy by evaluating tumors, lymphoid cell-rich organs, and immune-related adverse events using FDG-PET/CT. Clin Nucl Med. 2019;44:e272–e279. doi: 10.1097/RLU.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 19.Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. 2020;30:177–184. doi: 10.1089/thy.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anwar H, Sachpekidis C, Winkler J, Kopp-Schneider A, Haberkorn U, Hassel JC, et al. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45:376–383. doi: 10.1007/s00259-017-3870-6. [DOI] [PubMed] [Google Scholar]
- 21.Illouz F, Drui D, Caron P, Do CC. Expert opinion on thyroid complications in immunotherapy. Ann Endocrinol. 2028;79:555–561. doi: 10.1016/j.ando.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Medica. 2016;26:297–307. doi: 10.11613/BM.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr Oslo Nor. 2007;1992(96):644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43:121–137. doi: 10.1016/S0167-9473(02)00225-6. [DOI] [Google Scholar]
- 25.Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017;102:2770–2780. doi: 10.1210/jc.2017-00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS ONE. 2019;14:e0216954. doi: 10.1371/journal.pone.0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edo N, Morita K, Sakamoto M, Kaminaga T, Edo H, Okamura E, et al. Correlation between anti-thyroid peroxidase antibody levels and diffuse thyroid uptake of 18F-fluorodeoxyglucose in Hashimoto’s thyroiditis: a retrospective study. Thyroid Res. 2018;11:14. doi: 10.1186/s13044-018-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alhusseini M, Samantray J. Hypothyroidism in Cancer Patients on Immune Checkpoint Inhibitors with anti-PD1 Agents: Insights on Underlying Mechanisms. Exp Clin Endocrinol Diabetes. 2017;125:267–269. doi: 10.1055/s-0042-119528. [DOI] [PubMed] [Google Scholar]
- 29.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teulings H-E, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 32.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 34.Horvat TZ, Adel NG, Dang T-O, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang N, Dick J, Slynko A, Schulz C, Dimitrakopoulou-Strauss A, Sachpekidis C, et al. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy. 2019;11:667–676. doi: 10.2217/imt-2018-0146. [DOI] [PubMed] [Google Scholar]
- 36.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 37.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peiró I, Palmero R, Iglesias P, Díez JJ, Simó-Servat A, Marín JA, et al. Thyroid dysfunction induced by nivolumab: searching for disease patterns and outcomes. Endocrine. 2019;64:605–613. doi: 10.1007/s12020-019-01871-7. [DOI] [PubMed] [Google Scholar]
- 39.Basak EA, van der Meer JWM, Hurkmans DP, Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, et al. Overt thyroid dysfunction and anti- thyroid antibodies predict response to anti-PD-1 immunotherapy in cancer patients. Thyroid. 2020;30:966–973. doi: 10.1089/thy.2019.0726. [DOI] [PubMed] [Google Scholar]
- 40.Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for assessing tumour response to immunotherapy: report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging. 2019;46:238–250. doi: 10.1007/s00259-018-4171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Mushref M, Guido PA, Collichio FA, Moore DT, Clemmons DR. Thyroid dysfunction, recovery, and prognosis in melanoma patients treated with immune checkpoint inhibitors: a retrospective review. Endocr Pract. 2019;26:36–42. doi: 10.4158/EP-2019-0244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Histogram of maximum standardized uptake value in the thyroid (SUVmax-Th) and SUVmax-thyroid/SUVmax-blood-pool ratio (Th/B) in patients who developed permanent thyroid dysfunction (PTD) or not. file1 (TIFF 13125 kb)