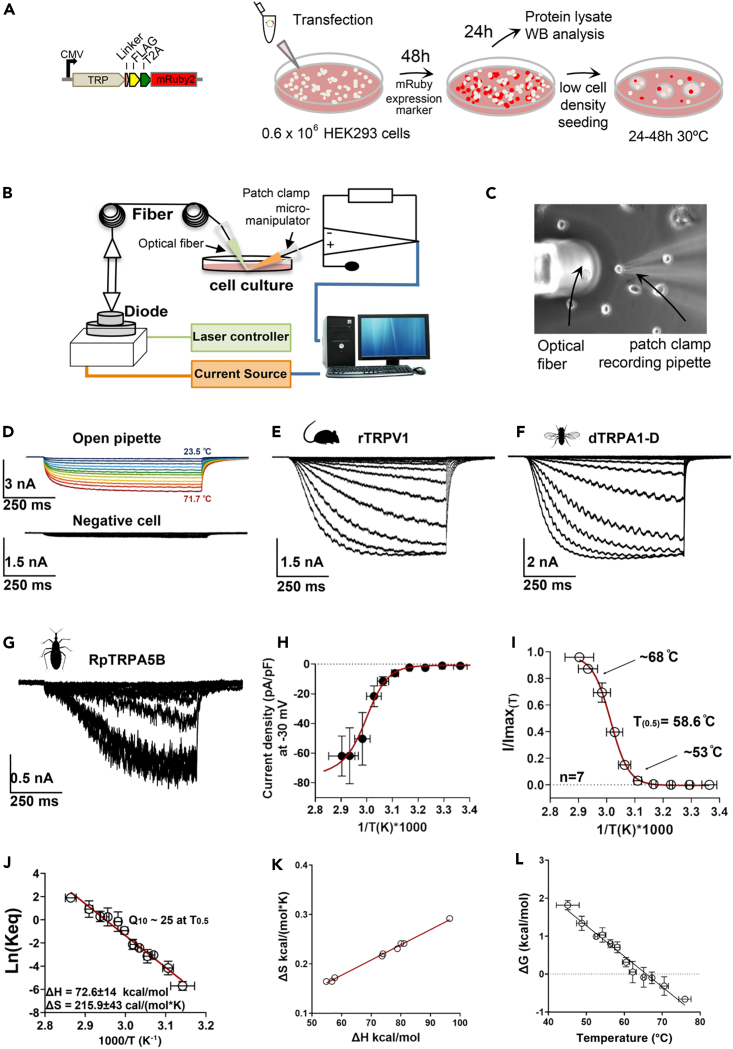
Figure 2.
Thermodynamics of RpTRPA5B temperature-activated currents
(A–C) Experimental workflow. (A) Each TRP channel subcloned in the pFRT-TO-FLAG-T2A-mRuby2 expression cassette51,100 was transfected in HEK293T cells seeded at low density and incubated at 37°C for 48 h. Cells were then prepared for patch-clamp recording by seeding in a 30-mm2 culture dish overlaid with round glass cover slips and incubated at 30°C. (B) Electrophysiology recordings took place after 24–48 h using an optical fiber-based setup adapted after Yao et al. 2010,52 designed to couple manual patch-clamp recordings with fiber optics as a way to provide controllable optical and thermal stimulations to individual cells expressing candidate thermosensitive receptor proteins. The setup consists of a fiber launch system combining a high-power optical fiber tuned to near-infrared wavelengths (λc = 1,460 nm (+/−20 nm), Po = 4.8 W), a visible alignment laser (red), and a laser diode controller, forming a PID control loop using the patch-clamp current as the feedback signal. (C) During the experiment, a laser spot is aligned with one single patched cell (see Figure S6) stably expressing the membrane receptor protein of interest in the coverslip placed in the recording chamber.
(D) Upper panel, current traces through the open patch-clamp pipette in response to temperature calibration steps from room temperature up to 71°C elicited by increments in the IR laser voltage input (see STAR Methods). Each 700 ms voltage pulse is represented in different colors for the different temperatures calculated from the open pipette currents. Lower panel, representative recording of non-transfected cells; these cells did not show robust temperature-elicited currents, like negative cells on the recording plate.
(E) Whole-cell currents evoked by temperature steps from HEK293T cells expressing rat TRPV1 (heat-activated mammalian vanilloid thermoTRP); cells were held at −30 mV during the recording.
(F) Whole-cell currents evoked by temperature steps from HEK293T cells expressing dTRPA1-D (holding potential of −30 mV). The sinusoidal pattern observed within the current curves is inherent to the cyclic modulation of the laser’s rapid “on-off” cycles.
(G) Whole-cell currents evoked by temperature steps in HEK293T cells expressing RpTRPA5B; cells were held at −30 mV.
(H) Current-temperature relationship for RpTRPA5B whole-cell current was normalized by cell membrane capacitance (current density); the red line corresponds to a modified Boltzmann function that includes the leak and unitary current temperature dependence (see STAR Methods).
(I) Fraction of RpTRPA5B channels in the open state (open probability, Po) as a function of the temperature. The Po vs. 1/T was fitted to a Boltzman function with the midpoint of activation (T0.5) reached at 58.6°C.
(J) van’t Hoff plot estimates of RpTRPA5B with an activation enthalpy of the endothermic transition at 92 kcal/mol and an entropic change associated with the temperature activation process at 274 cal/mol∗K at −30 mV.52
(K) Coupling between enthalpic (ΔH) and entropic (ΔS) changes for each one of the experiments recorded.
(L) Free energy (ΔG) associated with the activation process as a function of temperature for RpTRP5AB channels. The receptor activation is associated with small free energy changes, as reported before for other families of mammalian thermoTRP receptors. ΔG was calculated as -RT∗ln(Keq).72 Data are represented as mean ± standard error.