ABSTRACT
As higher-valent pneumococcal conjugate vaccines (PCVs) become available for pediatric populations in the US, it is important to understand healthcare provider (HCP) preferences for and acceptability of PCVs. US HCPs (pediatricians, family medicine physicians and advanced practitioners) completed an online, cross-sectional survey between March and April 2023. HCPs were eligible if they recommended or prescribed vaccines to children age <24 months, spent ≥25% of their time in direct patient care, and had ≥2 y of experience in their profession. The survey included a discrete choice experiment (DCE) in which HCPs selected preferred options from different hypothetical vaccine profiles with systematic variation in the levels of five attributes. Relative attribute importance was quantified. Among 548 HCP respondents, the median age was 43.2 y, and the majority were male (57.9%) and practiced in urban areas (69.7%). DCE results showed that attributes with the greatest impact on HCP decision-making were 1) immune response for the shared serotypes covered by PCV13 (31.4%), 2) percent of invasive pneumococcal disease (IPD) covered by vaccine serotypes (21.3%), 3) acute otitis media (AOM) label indication (20.3%), 4) effectiveness against serotype 3 (17.6%), and 5) number of serotypes in the vaccine (9.5%). Among US HCPs, the most important attribute of PCVs was comparability of immune response for PCV13 shared serotypes, while the number of serotypes was least important. Findings suggest new PCVs eliciting high immune responses for serotypes that contribute substantially to IPD burden and maintaining immunogenicity against serotypes in existing PCVs are preferred by HCPs.
KEYWORDS: Survey, discrete choice experiment, provider preferences, knowledge, attitudes, behaviors, pneumococcal conjugate vaccines, pneumococcal disease, pediatric vaccines
Introduction
Pneumococcal disease is caused by the gram-positive bacterium Streptococcus pneumoniae and can be classified as invasive, such as bacteremic pneumonia, bacteremia without focus, and meningitis, or noninvasive, such as non-bacteremic pneumococcal pneumonia and acute otitis media (AOM).1,2 The impact of pneumococcal conjugate vaccines (PCVs) in the United States (US) was reflected by the significant decrease in pneumococcal disease. From 1998 to 2019, there was a 93% reduction in prevalence among children <5 y old.3 Despite this reduction, certain vaccine-type serotypes, specifically 3, 19A, and 19F, continue to disproportionately contribute to disease burden in the US and S. pneumoniae is estimated to cause more than 300,000 deaths in children under the age of five globally each year.4,5
There are various pneumococcal vaccines that have been approved for use in pediatric populations in the US for the prevention of invasive pneumococcal disease. In 2000, the US Food and Drug Administration (FDA) approved a 7-valent PCV (PCV7) for the prevention of invasive pneumococcal disease (IPD) in infants aged <24 months and otitis media in infants and toddlers. In 2010, the FDA approved a 13-valent PCV (PCV13) for the prevention of IPD and otitis media (the otitis media indication covers the 7 serotypes included in PCV7) in children 6 weeks to 5 y old and the approval was expanded in January 2013 to include the prevention of IPD in children 6 to 17 y old.6,7 Most recently, in June 2022, PCV15 was approved for the prevention of IPD in infants and children 6 weeks through 17 y old and in April 2023,8 PCV20 was approved for the prevention of IPD in infants and children 6 weeks through 17 y old and for the prevention of otitis media (caused by the 7 serotypes included in PCV7) in infants 6 weeks through 5 y old.9
Previously, the Advisory Committee on Immunization Practices (ACIP) recommended PCV13 as the standard of care for US children, however, in June 2022, recommendations were amended to include both PCV13 and PCV15 and were amended again in June 2023 to include both PCV15 and PCV20 as vaccine options for children.8,10 For children <24 months, PCV15 and PCV20 are recommended for routine use according to the existing approved schedules.10
With multiple vaccine options available for use and the evolving prevention landscape in the US, it is increasingly important to understand health care providers’ (HCPs) knowledge, attitudes, and preferences regarding pneumococcal vaccination, especially considering that receiving information and a recommendation from HCPs has been associated with vaccine uptake.11,12 While there is existing literature on HCPs’ attitudes and preferences for pneumococcal vaccination in adults,13,14 little is known on HCPs’ attitudes and preferences for pneumococcal vaccination in children. The objective of this study was to assess HCPs preferences, and knowledge, attitudes, and behaviors (KAB) toward PCVs in children age <24 months in the United States to support the evaluation of the feasibility and acceptability of novel PCVs.
Materials and methods
Study design and eligibility criteria
A cross-sectional survey that included a discrete choice experiment (DCE), was conducted among HCPs (pediatricians, family medicine physicians, and advanced practitioners [nurse practitioners and physician assistants]) in the US between March 2023 and April 2023. A DCE is a research method used to quantify stakeholders’ preferences and understand key factors driving their decisions by systematically eliciting choices between hypothetical options (i.e., vaccine profiles).15 All HCPs were recruited by a recruitment vendor using its existing online physician panels. A random selection of potentially eligible HCPs (potentially eligible based on physician/clinician type, but may or may not meet all the study’s inclusion and exclusion criteria) were sent invitations and those HCPs who expressed interest in participating were screened for eligibility until the target sample size of eligible participants was reached. The sample size was based on power calculations for the DCE (which indicated that ~100 respondents would be needed for each provider type to allow for possible subgroup analysis). Thus, a target of 500 respondents across all HCP types was deemed sufficient to allow for some respondent loss due to failures of comprehension checks and still achieve the target sample size. To minimize bias, HCPs were blinded to the study sponsor. HCPs were paid an honorarium for their time that was determined to be within a fair market value range, considering time commitment and study type. The study was reviewed by an institutional review board (IRB) and was deemed exempt from IRB oversight.
HCPs were eligible if they 1) provided direct patient care to pediatric patients and recommend or administered vaccines as part of patient care, 2) worked in a practice that provided vaccines to infants and children aged ≤24 months, 3) spent at least 25% of their time providing care to pediatric patients, 4) practiced within the US (50 states and Washington DC), and 5) were able to complete the survey in English. HCPs were excluded from participating if they were unwilling to provide electronic agreement to participate in the online survey or had <2 y of experience in their profession.
Survey development
Qualitative, semi-structured exploratory interviews with HCPs who provided care to pediatric patients were conducted prior to survey development to identify possible vaccine attributes and concepts for inclusion in the DCE.16 Findings from a targeted literature review, exploratory interviews, and pretest interviews were used to inform the content of the survey. The survey included six sections: screener, electronic agreement form, demographic and practice characteristics, DCE attribute descriptions and comprehension checks, DCE choice tasks, and a KAB survey section. HCPs were also provided with a brief background on pneumococcal disease epidemiology. Comprehension check questions were constructed as simple exercises focusing exclusively on one attribute at a time to assess respondents’ comprehension of each attribute, such as by asking HCPs to select the level with the greatest number of serotypes. Two or more comprehension check failures, that is selecting a level with a lower number of serotypes or lower immune response as better than one with more serotypes or higher immune response, were an indicator of poor comprehension of the attributes and HCPs with two or more failures were disqualified from participating in the study.
A total of five vaccine attributes were examined in the DCE survey component: number of serotypes included in the vaccine, effectiveness against serotype 3, comparability of immune response for the serotypes shared with PCV13, percent coverage of IPD, and AOM label indication (Table 1). The combinations of attributes and levels that were presented to HCPs in the choice tasks were generated from the D-efficient Bayesian design using the Ngene software (version 1.2.1, by ChoiceMetrics). HCPs were instructed to assume similar cost and safety while reviewing hypothetical vaccine profiles. HCPs were presented with a total of 12 choice tasks, 10 of which were included in the analysis. Figure 1 shows an example of a possible choice task, in which each attribute is populated with a level and the participant would select the preferred hypothetical vaccine. The levels for each attribute were populated based on the survey design software and combinations were not forced; choice tasks may have resembled marketed products but were not designed specifically to do so. Of the other two, one was a repeated question to explore consistency, and the other was a dominance check with one choice superior on all attributes that served as a quality control measure. Failing the repeated question or dominance task did not automatically eliminate the HCPs from the preference analysis since the random utility model assumes an error component to account for seemingly irrational behavior.
Table 1.
Attributes and levels presented in the discrete choice experiment (DCE).
| Attributes | Levels and Descriptions |
|---|---|
| Number of serotypes included | PCVs can cover different numbers of serotypes. In this survey, we will ask you to consider PCVs covering the following numbers of serotypes, including:
|
| Effectiveness against serotype 3 | PCVs have different effectiveness against specific serotypes that varies by vaccine. This attribute, “Effectiveness Against Serotype 3,” describes effectiveness of the PCV against Serotype 3, which is the largest contributor to vaccine-type IPD. In this survey, we will ask you to consider the following effectiveness against serotype 3, including:
|
| Immune response for the serotypes covered in PCV13 | Immune response for specific serotypes varies among different PCVs; this refers to the effectiveness of newer vaccines for the 13 serotypes included in PCV13. In the survey, we will ask you to consider the following varying immune responses for the serotypes covered in PCV13, including:
|
| Percent Coverage of IPD | As different PCVs cover different serotypes, the proportion of IPD covered by the PCVs varies. In this survey, we will ask you to consider the following percent coverage of IPD, including:
|
| AOM label indication | A PCV may come with an indication for otitis media based on new or existing data, or without a disease-specific indication. In this survey, we will ask you to consider the following variations in indications, including:
|
Abbreviations: AOM: Acute otitis media; IPD: Invasive pneumococcal disease; PCV: Pneumococcal conjugate vaccine.
Figure 1.
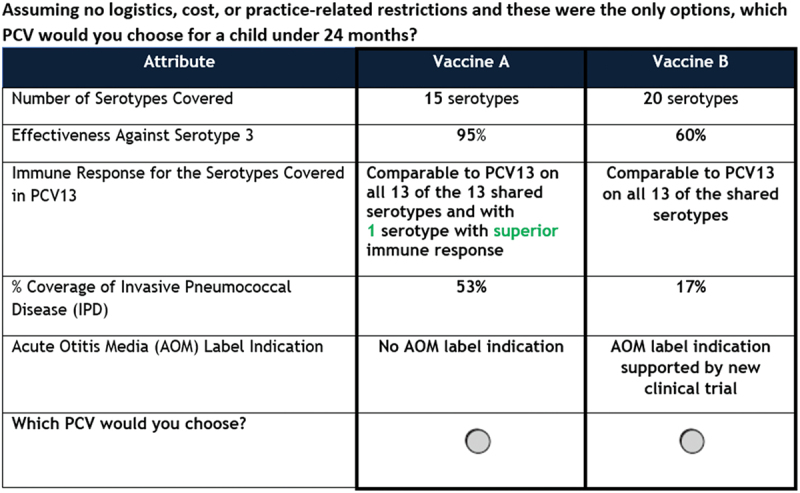
Example of choice task in discrete choice experiment.
Abbreviations: AOM: Acute otitis media; IPD: Invasive pneumococcal disease; PCV: Pneumococcal conjugate vaccine.
Data collection and study measures
Survey links were distributed via e-mail to verified HCP panelists who met screening criteria. HCPs interested in participating in the survey answered a series of questions via self-report to confirm eligibility before the start of the survey, complementing the panel’s enrollment and ongoing validation processes. Data collected included HCP demographics and practice characteristics, DCE choice task selections, and KAB regarding currently available PCVs, new PCVs, immunogenicity creep, and breakthrough disease. Immunogenicity creep was defined as the potential reduction in immune response as more serotypes are added to expanded valency PCVs17 and breakthrough disease was defined as “disease caused by vaccine-type serotypes following partial vaccination.”
Data analysis
HCP demographics and practice characteristics were analyzed descriptively using univariate statistics. Mean, standard deviation, median, and range are presented for continuous variables while frequency and percent are presented for categorical variables.
Dummy-coded conditional logit regression models were used to examine the observed choice task responses in the form of odds ratios (ORs). Relative attribute importance (RAI) scores, the proportion of total variance explained by individual attributes expressed as a percentage, were computed to understand the importance HCPs place on each of the attributes. The higher the score, the greater the influence of that attribute on decision-making. Sensitivity analysis was conducted to compare results for the study sample with and without failures (i.e., excluding respondents who failed one comprehension check or the dominance question). Data analyses were conducted using Stata® 14.2 (for the analysis of the DCE) and SAS/STAT® 14.3 (for the remainder of analyses of survey data).
Results
Demographic and practice characteristics of HCPs
A total of 32 respondents failed 2 or more comprehension checks, and thus, were disqualified from participating in the study. A total of 548 remaining HCPs (332 physicians and 216 advanced practitioners) met the inclusion criteria and completed the survey. HCPs were an average of 43.2 y old, and a majority were male (57.9%), White (77.4%), and practiced in an urban location (69.7%). Hospital-owned practices were the most common settings (38.7%) and most practices/institutions had a program to incentivize childhood vaccination (53.5%). HCPs reported that more than half of their patients (50.8%) are covered under public insurance and are without chronic medical conditions (50.9%) (Table 2).
Table 2.
HCP and practice characteristics.
| Variable | Statistic or Category | All (n = 548) |
Physicians (n = 332) |
Advanced practitioners (n = 216) |
|---|---|---|---|---|
| Professional background, n (%) | Physician – Family Medicine | 170 (31.0%) | 170 (51.2%) | - |
| Physician – Pediatrician | 162 (29.6%) | 162 (48.8%) | - | |
| Nurse Practitioner | 108 (19.7%) | - | 108 (50.0%) | |
| Physician Assistant | 108 (19.7%) | - | 108 (50.0%) | |
| Age (Years) | Mean (SD) | 43.2 (8.1) | 44.9 (8.5) | 40.6 (6.5) |
| Median (Q1 to Q3) | 41 (38 to 46) | 43 (39 to 48) | 40 (36 to 44) | |
| Range | 24 to 76 | 30 to 76 | 24 to 64 | |
| Gender, n (%) | Male | 317 (57.9%) | 219 (66.0%) | 98 (45.4%) |
| Race*b, n (%) | White | 424 (77.4%) | 248 (74.7%) | 176 (81.5%) |
| Black or African American | 59 (10.8%) | 36 (10.8%) | 23 (10.7%) | |
| Asian | 49 (8.9%) | 37 (11.1%) | 12 (5.6%) | |
| Othera | 13 (2.4%) | 8 (2.4%) | 5 (2.3%) | |
| Prefer not to answer | 3 (0.6%) | 3 (0.9%) | 0 (0.0%) | |
| Practice Location, n (%) | Urban | 382 (69.7%) | 217 (65.4%) | 165 (76.4%) |
| Suburban | 157 (28.7%) | 108 (32.5%) | 49 (22.7%) | |
| Rural | 9 (1.6%) | 7 (2.1%) | 2 (0.9%) | |
| Number of pediatric patients (age<24 months) HCP personally sees per month, n (%) | <10 | 62 (11.3%) | 41 (12.4%) | 21 (9.7%) |
| 10–20 | 57 (10.4%) | 33 (9.9%) | 24 (11.1%) | |
| 20–50 | 228 (41.6%) | 137 (41.3%) | 91 (42.1%) | |
| 50–100 | 193 (35.2%) | 119 (35.8%) | 74 (34.3%) | |
| >100 | 8 (1.5%) | 2 (0.6%) | 6 (2.8%) | |
| Years in practice since completion of residency | Mean (SD) | 12.5 (6.6) | 14.2 (6.9) | 9.9 (4.7) |
| Median (Q1 to Q3) | 11 (8 to 15) | 12 (10 to 17) | 9 (7 to 12) | |
| Range | 2 to 39 | 3 to 39 | 2 to 36 | |
| Type of practiceb, n (%) | Solo/duo practice | 134 (24.5%) | 100 (30.1%) | 34 (15.7%) |
| Group practice | 197 (36.0%) | 131 (39.5%) | 66 (30.6%) | |
| Hospital owned | 212 (38.7%) | 106 (31.9%) | 106 (49.1%) | |
| Federally Qualified Health Center | 75 (13.7%) | 34 (10.2%) | 41 (19.0%) | |
| Academic health center | 76 (13.9%) | 38 (11.5%) | 38 (17.6%) | |
| Integrated delivery network | 11 (2.0%) | 5 (1.5%) | 6 (2.8%) | |
| Institution incentivized to improve immunization rate*, n (%) | Yes, via pay for performance or quality indicators | 293 (53.5%) | 161 (48.5%) | 132 (61.1%) |
| No | 212 (38.7%) | 144 (43.4%) | 68 (31.5%) | |
| Don’t know | 43 (7.9%) | 27 (8.1%) | 16 (7.4%) | |
| Estimated insurance mix of patients*, mean | Public insurance | 50.8% | 48.6% | 54.3% |
| Private insurance | 37.7% | 40.2% | 33.9% | |
| Self-pay | 11.5% | 11.2% | 11.9% | |
| Percentage of pediatric patients (age<24 months) with underlying conditions, mean | No chronic conditions | 50.9% | 62.3% | 33.4% |
| Cerebrospinal fluid leak | 5.4% | 3.7% | 8.0% | |
| Cochlear implant | 5.6% | 4.0% | 8.0% | |
| Diabetes | 6.5% | 4.7% | 9.1% | |
| Immunocompromised (such as HIV, cancer, taking immunosuppressants, asplenia) | 6.9% | 4.7% | 10.2% | |
| Sickle cell disease | 6.0% | 4.1% | 8.9% | |
| Severe asthma | 5.4% | 4.2% | 7.3% | |
| Other chronic condition(s) | 13.3% | 12.3% | 15.1% |
Abbreviations: HCP: Healthcare provider; HIV: Human immunodeficiency virus; SD: Standard deviation; Q1: First quartile; Q3: Third quartile.
*Due to rounding, values do not add up to 100.0%.
aOther includes the following categories: American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and Middle Eastern or North African.
bMultiple options allowed.
Knowledge, attitudes, and behaviors of HCPs regarding PCVs
Most HCPs (92.3%) agreed or strongly agreed that they would like to have more approved options for patients <24 months old, with the most common reasons being flexibility to select a new PCV option based on immune responses (58.1%) and number of serotypes covered (52.8%). Most HCPs reported that they were familiar (extremely [23.5%], moderately [46.4%), or somewhat [24.6%]) with higher-valent PCVs that are newly approved or in development for pediatric patients <24 months old (Table 3).
Table 3.
Knowledge, attitudes, and behaviors of HCPs regarding PCVs.
| Variable | Statistic or Category | HCPs (n = 548) |
|---|---|---|
| I would like to have more approved PCV options for my pediatric patients (age <24 months)*, n (%) | Strongly agree | 240 (43.8%) |
| Agree | 266 (48.5%) | |
| I’m not sure/undecided | 33 (6.0%) | |
| Disagree | 9 (1.6%) | |
| Strongly disagree | 0 (0.0%) | |
| Reasons for wanting more approved PCV options (if Strongly Agree or Agree to more approved PCV options)a, n (%) | Can decide based on no. of serotypes covered | 267 (52.8%) |
| Can decide based on immune response | 294 (58.1%) | |
| Can decide based on disease specific indication | 224 (44.3%) | |
| Can decide based on patient characteristics | 249 (49.2%) | |
| Can decide based on price | 135 (26.7%) | |
| Less concern about supply and shipment | 39 (7.7%) | |
| Reasons for not wanting more approved vaccines (if Strongly Disagree or Disagree)b, n (%) | Current vaccines work well enough | 5 (55.6%) |
| Hard to convince families | 1 (11.1%) | |
| Too confusing for ordering and billing | 5 (55.6%) | |
| Multiple options can create logistical and administrative issues | 6 (66.7%) | |
| Familiarity with higher valent PCVs that are newly approved or under development for pediatric patients (age < 24 months), n (%) | Not at all familiar | 2 (0.4%) |
| Slightly familiar | 28 (5.1%) | |
| Somewhat familiar | 135 (24.6%) | |
| Moderately familiar | 254 (46.4%) | |
| Extremely familiar | 129 (23.5%) | |
| Familiarity with “Immunogenicity Creep,” n (%) | I am familiar with the concept | 279 (50.9%) |
| I am somewhat familiar with the concept | 196 (35.8%) | |
| I am not familiar with the concept | 73 (13.3%) | |
| Concern for immunogenicity creep, n (%)* | Not at all concerned | 66 (12.0%) |
| Slightly concerned | 142 (25.9%) | |
| Somewhat concerned | 170 (31.0%) | |
| Moderately concerned | 121 (22.1%) | |
| Extremely concerned | 49 (8.9%) | |
| Concern for breakthrough disease with newer PCVs that include a larger number of serotypes, n (%)* | Not at all concerned | 61 (11.1%) |
| Slightly concerned | 132 (24.1%) | |
| Somewhat concerned | 186 (33.9%) | |
| Moderately concerned | 119 (21.7%) | |
| Extremely concerned | 50 (9.1%) |
Abbreviations: HCP: Healthcare provider; PCV: Pneumococcal conjugate vaccine.
aOnly HCPs who responded “strongly agree” or “agree” (n = 506) to question: “How much do you agree or disagree with the following statement? I would like to have more approved PCV options for my pediatric patients (age <24 months),” provided reasons for wanting more approved PCV options. HCPs were able to select more than one reason.
bOnly HCPs who responded “strongly disagree” or “disagree” (n = 9) to question: “How much do you agree or disagree with the following statement? I would like to have more approved PCV options for my pediatric patients (age <24 months),” provided reasons for not wanting more approved PCV options. HCPs were able to select more than one reason.
*Due to rounding, values do not add up to 100.0%.
Among different information sources used in deciding to recommend a PCV, CDC recommendations (18.2%), information from the manufacturer (12.6%), and published clinical studies (12.5%) were considered the most important in the decision-making process (Figure 2).
Figure 2.
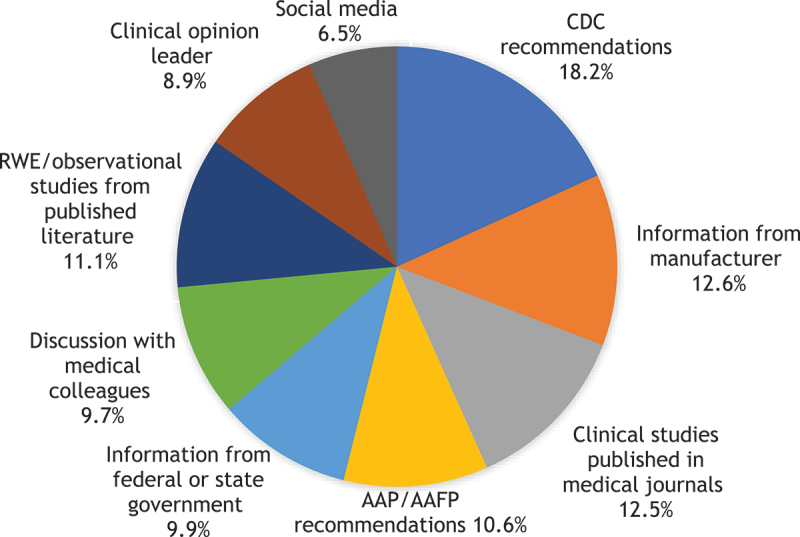
Most important information source in making PCV recommendations (n = 548).
Abbreviations: AAP: American Academy of Pediatrics; AAFP: American Academy of Family Practitioners; CDC: Centers for Disease Control and Prevention; PCV: Pneumococcal conjugate vaccine; RWE: Real-world evidence.
Approximately half of the HCPs (50.9%) were already familiar with the concept of immunogenicity creep. Moreover, 62.0% of HCPs reported being at least somewhat concerned about immunogenicity creep (extremely [8.9%], moderately [22.1%], and somewhat [31.0%]) and 64.8% of HCPs were at least somewhat concerned for breakthrough disease with new higher-valent PCVs (extremely [9.1%], moderately [21.7%], and somewhat [33.9%]) (Table 3).
DCE choice task preferences of HCPs
Analyses were conducted for all respondents and excluding respondents who failed a single comprehension check (n = 34) or dominance question (n = 28). There were no statistical differences, so all respondents were retained in the analysis. RAI was highest for immune response for the shared serotypes covered in PCV13 (RAI score: 31.4%), followed by percent coverage of IPD (21.3%), AOM indication (20.3%), effectiveness against serotype 3 (17.6%), and number of serotypes included (9.5%) (Figure 3).
Figure 3.
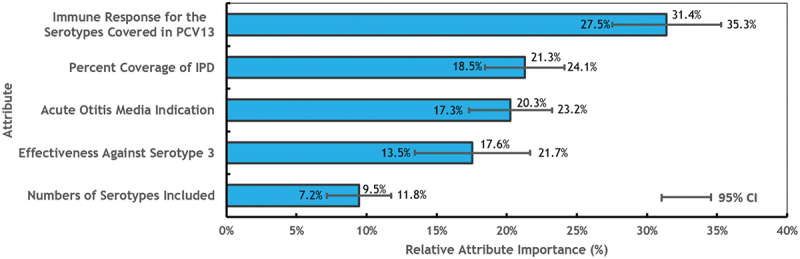
Relative attribute importance (RAI) of PCV attributes estimated from regression models (n = 548).
Abbreviations: CI: Confidence interval; IPD: Invasive pneumococcal disease; PCV: Pneumococcal conjugate vaccine; RAI: Relative attribute importance.
HCPs were three times (OR: 3.0, 95% confidence interval [CI]: 2.5–3.6) as likely to prefer a vaccine with an immune response comparable to PCV13 on all 13 of 13 serotypes with 1 superior serotype, compared to a vaccine with an immune response comparable to PCV13 on 8 of 13 serotypes and inferior on the remaining 5 serotypes. They were also 2.1 (95% CI: 1.8–2.4) times as likely to prefer 53% coverage of IPD compared to 17% coverage and 2.0 (95% CI: 1.8–2.3) times as likely to prefer a vaccine with an AOM label indication based on data from new clinical trials compared to a vaccine with no AOM label indication. HCPs were also 1.4 (95% CI: 1.3–1.5) times as likely to prefer a PCV vaccine that covers 20 serotypes compared to a vaccine that covers 13 serotypes and 1.9 (95% CI: 1.6–2.2) times as likely to prefer a vaccine with 95% effectiveness against serotype 3 compared to 15% effectiveness (Table 4). HCPs’ preferences for effectiveness behaved in a stair-step pattern, with a trend for stronger preferences for high levels of effectiveness, although confidence intervals often overlapped.
Table 4.
Odds ratio preference results estimated from regression models (n = 548).
| Variable | Odds Ratio (OR) | 95% CI | P Value | |
|---|---|---|---|---|
| Number of Serotypes Included | 13 serotypes | Reference | Reference | |
| 15 serotypes | 1.1 | (1.0, 1.2) | .01 | |
| 20 serotypes | 1.4 | (1.3, 1.5) | <.001 | |
| Effectiveness Against Serotype 3 | 15% | Reference | Reference | |
| 30% | 1.1 | (1.0, 1.2) | .07 | |
| 60% | 1.4 | (1.2, 1.6) | <.001 | |
| 80% | 1.8 | (1.5, 2.1) | <.001 | |
| 95% | 1.9 | (1.6, 2.2) | <.001 | |
| Immune Response for the Serotypes Covered in PCV13 | Comparable to PCV13 on 8 of the 13 shared serotypes with 5 serotypes with inferior immune response | Reference | Reference | |
| Comparable to PCV13 on 10 of the 13 shared serotypes with 3 serotypes with inferior immune response | 1.1 | (1.0, 1.3) | .17 | |
| Comparable to PCV13 on all 13 of the shared serotypes | 1.7 | (1.5, 2.0) | <.001 | |
| Comparable to PCV13 on all 13 of the 13 shared serotypes with 1 serotype with superior immune response | 3.0 | (2.5, 3.6) | <.001 | |
| Percent Coverage of IPD | Covers serotypes accounting for 17% of the current residual IPD burden | Reference | Reference | |
| Covers serotypes accounting for 38% of the current residual IPD burden | 1.6 | (1.4, 1.8) | <.001 | |
| Covers serotypes accounting for 53% of the current residual IPD burden | 2.1 | (1.8, 2.4) | <.001 | |
| Acute Otitis Media (AOM) indication | No AOM indication | Reference | Reference | |
| AOM indication supported by AOM efficacy data from PCV7; no data on efficacy of newer, non-PCV7 serotypes to AOM | 1.7 | (1.6, 1.9) | <.001 | |
| AOM indication supported by a clinical trial specific to the new vaccine | 2.0 | (1.8, 2.3) | <.001 |
Abbreviations: AOM: Acute otitis media; CI: Confidence interval; HCP: Healthcare provider; IPD: Invasive pneumococcal disease; OR: Odds ratio; PCV: Pneumococcal conjugate vaccine; SD: Standard deviation; Q1: First quartile; Q3: Third quartile.
Discussion
To our knowledge, this is the first DCE survey study to assess the knowledge, attitudes, behaviors, and preferences of US HCPs regarding PCVs in children aged <24 months. Results demonstrated that almost all HCPs were interested in having more approved PCV options for younger patients (<24 months). Most HCPs also reported concern about immunogenicity creep and breakthrough disease with newer higher-valent PCVs. Moreover, CDC recommendations were the most important information source influencing HCP decision-making regarding PCV recommendations.
In the DCE, immune response for the shared PCV13 serotypes was considered the most important factor in HCPs’ decision-making for PCVs, while the number of serotypes was considered least important. The relatively lower importance for the number of serotypes compared with attributes describing immune response and disease burden suggests that respondents are aware that serotypes do not equally contribute to the burden of disease. Results demonstrate that most HCPs in this study believe that maintaining comparable immune response for PCV13 vaccine-type serotypes, which are estimated to cause 37.4% of global IPD burden in children <5 y old,18 is more important than expanding the number of serotypes.
Within each individual attribute included in the DCE choice tasks, there was a clear and expected pattern in level preferences, even though there were not always significant differences between adjacent levels. Overall, HCPs’ responses were logical and indicated that when they considered the levels of each attribute individually, they preferred a stronger immune response, higher effectiveness against serotype 3, a higher percentage of coverage against IPD, an AOM indication, and a higher number of included serotypes. It is important to note that there was no significant difference in preferences for the source of evidence (new clinical trial data vs. immunobridging data from PCV7) used for an AOM label indication, however, our findings indicate that having an AOM indication is significantly valuable to HCPs, which may be due to experience with AOM as the most common infectious disease in children <24 months old.19
While there are no other published preference studies among US HCPs regarding PCVs in children <24 months old, studies related to other vaccines can provide some insights on vaccine preferences, although vaccine attributes are not defined identically. A multi-country preference study of vaccine-related attitudes and concerns of HCPs who provide pediatric care found that severity of the relevant disease and inclusion of the vaccine in formal vaccination schedules were the most important factors affecting HCPs pediatric vaccine recommendations.20 Another study in China on HCPs’ preferences regarding COVID-19 vaccines for pediatric patients found that HCPs considered vaccine efficacy to be the most influential attribute on their preferences, when compared to vaccine safety and number of doses administered.21 Moreover, a study on US pediatricians’ preferences for hypothetical meningococcal vaccines for infants found increases in vaccine effectiveness to be one of the most important factors influencing their recommendation.22 Insights from other non-PCV specific preference studies corroborate the findings of our study that coverage of more severe disease (such as IPD) and vaccine effectiveness may heavily influence HCP decision-making regarding pediatric vaccinations.
This study was subject to limitations. First, HCPs were recruited from online panels and HCPs who participate in online panels may differ from those who do not, potentially reducing the generalizability of our results. Second, the HCP sample was predominantly White (77%), male (58%), and worked in an urban location (70%), which may not be representative of the US pediatric HCP population (less than 40% male according to the American Board of Pediatrics), nor are they likely to be representative of HCP populations outside the US. Social desirability bias may also have affected results; if HCPs were uncomfortable self-reporting limited knowledge of PCVs, they may falsely report higher knowledge or familiarity with PCVs. However, this bias was mitigated by using an online survey completed independently and confidentially. Third, the DCE required that respondents evaluated hypothetical profiles and assumed comparable safety and cost when comparing vaccine profiles, all of which may or may not reflect real-world vaccine profiles. However, qualitative work and pre-testing were undertaken to ensure that the vaccine profiles were clinically relevant and reflected characteristics of vaccines currently available and in development. Lastly, subgroup analyses were not conducted to explore whether there were different preferences for vaccine attributes across subpopulations of HCPs, which could serve as effect modifiers for our results.
This study also has several strengths. First, results help to fill an important knowledge gap about the importance of characteristics of new PCVs to HCPs as new PCVs are being introduced and incorporated into pediatric pneumococcal vaccine recommendations. While there are existing preference studies on other vaccines, none have specifically considered attributes of pediatric PCVs. Through a literature search and a qualitative phase in which we interviewed all four types of HCPs included in this study (pediatricians, family medicine physicians, nurse practitioners, and physician assistants), we developed a set of attributes and levels that were clinically relevant and can inform discussions about preferences and acceptability of newer PCVs.
Conclusion
This study found that comparability of immune response for shared serotypes covered in PCV13 and percentage coverage of IPD were the most important factors affecting HCPs’ decision to recommend a PCV, while the number of serotypes was deemed to be least important among the attributes explored. These findings suggest new PCVs eliciting high immune responses for serotypes that contribute substantially to IPD burden and maintaining immunogenicity against serotypes in existing PCVs, are preferred by HCPs.
Acknowledgments
The authors would like to thank all HCPs who participated in this study, as well as Empanel for its assistance in recruiting participants for this study. Data analysis support provided by Debdeep Chattopadhyay from OPEN Health, Rotterdam, the Netherlands.
Funding Statement
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Disclosure statement
S.M., J.W., K.A.F., B.C., and T.W.W. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in Merck & Co., Inc., Rahway, NJ, USA. Inc. J.T, N.N, A.P, R.P.V, M.H and J.K.S are employees of OPEN Health, which received consulting fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
References
- 1.Scelfo C, Menzella F, Fontana M, Ghidoni G, Galeone C, Facciolongo NC.. Pneumonia and invasive pneumococcal diseases: the role of pneumococcal conjugate vaccine in the era of multi-drug resistance. Vaccines (Basel). 2021. Apr 22;9(5):420. doi: 10.3390/vaccines9050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012. Jul;25(3):409–9. doi: 10.1128/CMR.00018-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . Surveillance and reporting; 2020. Sep 1 [accessed 2023 Jan 5]. https://www.cdc.gov/pneumococcal/surveillance.html.
- 4.Centers for Disease Control and Prevention . Global Pneumococcal disease and vaccination; 2022. Jan 27 [accessed 2023 Jan 5]. https://www.cdc.gov/pneumococcal/global.html.
- 5.Gierke R. Current epidemiology of pediatric pneumococcal disease, United States. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/Pneumococcal-02-Gierke-508.pdf.
- 6.Pfizer . Pfizer receives FDA approval for prevnar 13™ for the prevention of invasive pneumococcal disease in infants and young children; 2010. Feb 24 [accessed 2023 Jan 5]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_fda_approval_for_prevnar_13_for_the_prevention_of_invasive_pneumococcal_disease_in_infants_and_young_children.
- 7.Centers for Disease Control and Prevention (CDC) . Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62(25):521–524. [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi M, Farrar JL, Gierke R, Leidner AJ, Campos-Outcalt D, Morgan RL, Long SS, Poehling KA, Cohen AL, Poehling KA, et al. Use of 15-Valent Pneumococcal conjugate vaccine among U.S. children: updated recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174–1181. doi: 10.15585/mmwr.mm7137a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfizer . U.S. FDA approves PREVNAR 20®, Pfizer’s 20-valent pneumococcal conjugate vaccine for infants and children; 2023. Apr 27 [accessed 2023 June 7]. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-prevnar-20r-pfizers-20-valent-pneumococcal.
- 10.Centers for Disease Control and Prevention . Advisory committee on immunization practices (ACIP); 2022 Jan 28 [accessed 2023 Jan 5]. https://www.cdc.gov/vaccines/acip/index.html.
- 11.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Sources and perceived credibility of vaccine-safety information for parents. Pediatrics. 2011;127(Suppl 1):107–112. doi: 10.1542/peds.2010-1722P. [DOI] [PubMed] [Google Scholar]
- 12.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122(4):718–725. doi: 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 13.Vietri J, Meyers K, Poulos C, Chilson E, Sweeney C, Davis K, Snow V. United States healthcare provider preferences for adult pneumococcal vaccine recommendations. Open Forum Infect Dis. 2021. Dec 4;8(Suppl 1):S127. doi: 10.1093/ofid/ofab466.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco P, Myers K, Poulos C, Sweeney C, Hollis K, Snow V, Vietri JT. Preferences for adult Pneumococcal vaccine recommendations among United States health care providers. Infect Dis Ther. 2019;8(4):657–670. doi: 10.1007/s40121-019-00266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty S, Tsai J-H, Ning N, Pena-Molina A, Verma RP, Heisen M, Weaver J, Feemster KA, Weiss T, Schmier J, et al. 583. Preferences and attitudes of healthcare providers towards pneumococcal conjugate vaccines (PCVs) for children ages two and under in the United States (US). Open Forum Infect Dis. 2022;9(Supplement_2). doi: 10.1093/ofid/ofac492.635. [DOI] [Google Scholar]
- 17.Kwambana-Adams BA, Mulholland EK, Satzke C; ISPPD Group . State-of-the-art in the pneumococcal field: proceedings of the 11th international symposium on pneumococci and pneumococcal diseases (ISPPD-11). Pneumonia (Nathan). 2020. Feb 5;12:2. doi: 10.1186/s41479-019-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant LR, Slack MPE, Theilacker C, Vojicic J, Dion S, Reinert R-R, Jodar L, Gessner BD. Distribution of serotypes causing invasive pneumococcal disease in children from high-income countries and the impact of pediatric pneumococcal vaccination. Clin Infect Dis. 2023;76(3):1062–1070. doi: 10.1093/cid/ciac475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jamal A, Alsabea A, Tarakmeh M, Safar A. Etiology, diagnosis, complications, and management of acute otitis media in children. Cureus. 2022. Aug 15;14(8):e28019. doi: 10.7759/cureus.28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakhache P, Rodrigo C, Davie S, Ahuja A, Sudovar B, Crudup T, Rose M. Health care providers’ and parents’ attitudes toward administration of new infant vaccines—a multinational survey. Eur J Pediatr. 2013;172(4):485–492. doi: 10.1007/s00431-012-1904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Z, Song K, Wang Q, Zang S, Tu S, Chantler T, Larson HJ. Childhood COVID-19 vaccine acceptance and preference from caregivers and healthcare workers in China: a survey experiment. Prev Med. 2022;161:107138. doi: 10.1016/j.ypmed.2022.107138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulos C, Reed Johnson F, Krishnarajah G, Anonychuk A, Misurski D. Pediatricians’ preferences for infant meningococcal vaccination. Value Health. 2015;18(1):67–77. doi: 10.1016/j.jval.2014.10.010. [DOI] [PubMed] [Google Scholar]


