Abstract
Objectives:
To evaluate the benefit of a prototype circulation time-based test bolus evaluation algorithm for the individualized optimal timing of contrast media (CM) delivery in patients undergoing coronary CT angiography (CCTA).
Methods:
Thirty-two patients (62 ± 16 years) underwent CCTA using a prototype bolus evaluation tool to determine the optimal time-delay for CM administration. Contrast attenuation, signal-to-noise ratio (SNR), objective, and subjective image quality were evaluated by two independent radiologists. Results were compared to a control cohort (matched for age, sex, body mass index, and tube voltage) of patients who underwent CCTA using the generic test bolus peak attenuation +4 s protocol as scan delay.
Results:
In the study group, the mean time delay to CCTA acquisition was significantly longer (26.0 ± 2.9 s) compared to the control group (23.1 ± 3.5 s; p < 0.01). In the study group, SNR improvement was seen in the right coronary artery (17.5 vs 13; p = 0.028), the left main (15.3 vs 12.3; p = 0.027), and the left anterior descending artery (18.5 vs 14.1; p = 0.048). Subjective image quality was rated higher in the study group (4.75 ± 0.7 vs 3.64 ± 0.5; p < 0.001).
Conclusions:
The prototype test bolus evaluation algorithm provided a reliable patient-specific scan delay for CCTA that ensured homogenous vascular attenuation, improvement in objective and subjective image quality, and avoidance of beam hardening artifacts.
Advances in knowledge:
The prototype contrast bolus evaluation and optimization tool estimated circulation time-based time-delay improves the overall quality of CCTA.
Introduction
Coronary computed tomography angiography (CCTA) has become the examination of choice for non-invasive imaging of the coronary arteries. 1–3 The assessment of the coronary arteries for atherosclerotic disease relies heavily on image quality and contrast conditions. Optimal timing of contrast media (CM) administration is crucial to fully utilize the advantages of CCTA. With increasing scan speed achieved by advanced CT scanners, this task is becoming more complex. 4–6
In clinical routine, bolus tracking and test bolus are the most frequently used techniques for the determination of the timing of CCTA acquisition post CM administration. The bolus tracking technique based on a single contrast injection for patient-specific timing adjustment is utilized in the majority of clinical scenarios. Conversely, the test bolus technique allows for prospectively planning the shape of the time-attenuation curve to improve signal homogeneity. 5 Usually, contrast timing is derived based upon a standard 20-ml test bolus injection. However, the time-to-peak enhancement of the test bolus and the diagnostic scan may differ significantly due to the amount of CM administered. In many clinical institutions, the time-to-peak enhancement derived from the test bolus scan leads to the selection of a generic +4 s time delay. 5,7,8 To date, there is however no gold standard for a specific time delay in CCTA and recent SCCT guidelines suggest a range of +2 to+4 s. 9 Previous simulation studies performed on a physiological compartment model were able to simulate the patient- and organ-specific enhancement curves for a given CM injection, 10,11 and predict the required injection protocol to achieve optimized contrast enhancement. 12 However, those studies have not been tested in a clinical setting.
A prototype bolus evaluation tool has been developed to optimize CM delivery using simulated contrast dynamics for given test bolus information. The purpose of this study was to prospectively evaluate the utility of this novel test bolus evaluation tool for the individualized optimal timing of CM delivery in patients undergoing CCTA.
Methods and materials
Study design
This prospective, single-center, HIPAA-compliant study was approved by the local Institutional Review Board and all patients provided written informed consent. Consecutive patients referred for clinically indicated CCTA for evaluation of suspected coronary artery disease were enrolled between February 2019 and August 2019. Exclusion criteria included: glomerular filtration rate below 45 mL/min, respiratory impairment, and unstable clinical status. A matched control cohort was retrospectively assembled to evaluate image quality parameters of CCTA using time to test bolus peak attenuation +4 s as scan delay. Patients were matched based upon patient age, sex, body weight, and tube voltage.
Coronary CT angiography
CCTA examinations were performed on a third-generation dual-source CT system (SOMATOM Force, Siemens Healthineers, Forchheim, Germany). Prospective electrocardiography (ECG)-triggering was used for all CCTA acquisitions. Beta-blockers were typically administered before scanning with a target heart rate as close to 60 beats per minute (bpm) as possible. Sublingual nitroglycerin (0.4 mg) was administered before CCTA to patients with no contraindication.
A topogram of the chest was obtained to determine z-coverage for the CCTA scan. CM volume and injection rate for the test bolus were 20 ml and 4 ml s−1, respectively. A biphasic contrast injection protocol was used for the main CCTA scan to administer 1.0 ml per kg body weight (maximum 120 ml) of CM (iopromide, Ultravist, 370 mg of iodine per mL, Bayer, Indianola, PA) at 4–6 ml s−1, followed by 25 ml of CM diluted with 25 ml of saline chaser at the same rate. All images were acquired during inspiratory breath-hold.
The main scanning parameters were as follows: tube potential automatically selected using an automated tube voltage selection algorithm (CARE kV, Siemens Healthineers), 70–130 kV, tube current–time product, 200–650 mAs; gantry rotation time, 0.25 s; detector collimation, 2 × 192 × 0.6 mm. The CT dose index (CTDI) and dose length product (DLP) were obtained from patients’ records.
Test bolus technique
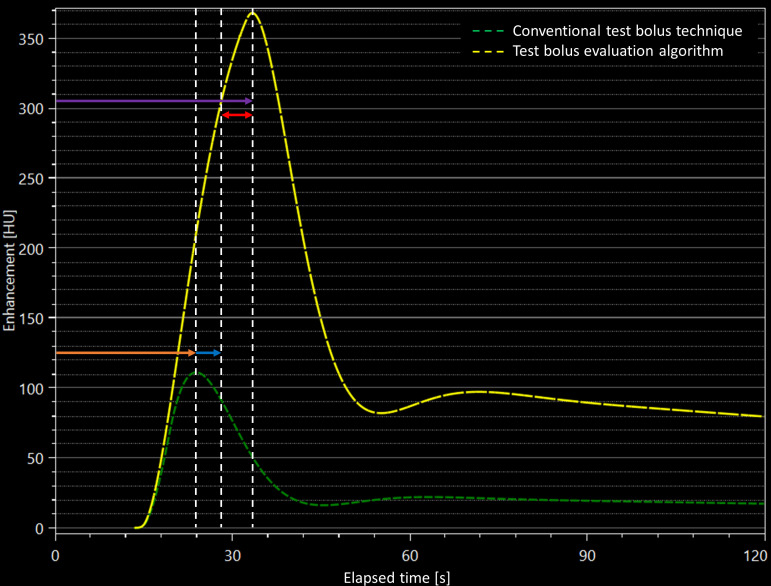
The test bolus technique was based on the i.v. injection of 20-mL CM during the acquisition of a series of dynamic low-dose (120 kV, 20 mAs) monitoring scans at the level of the ascending aorta. Monitoring scans were stared 10 s after the beginning of the injection of i.v. CM with a 1 s delay between each monitoring scan acquisition. In the control group, the time to peak enhancement was calculated and a + 4 s delay was applied prior to the start of the CCTA scan. In the study group, a dedicated prototype software (Bolus Evaluation, Siemens) was used to analyze the test bolus enhancement curve and calculate the time to peak enhancement for the CCTA acquisition depending on individual circulation time. Figure 1 shows a representative example for the difference between the standard and the prototype-based CCTA timing.
Figure 1.
Representative time-attenuation plot showing the calculation of scan delay using the conventional test bolus technique (green dashed curve) and the test bolus evaluation algorithm (yellow dashed curve). In this subject, peak enhancement was measured at 24 s (orange arrow) after contrast bolus administration. Using the generic +4 s time delay (blue arrow), the total time delay before the start the CCTA would be 28 s. On the other hand, the simulated peak enhancement was achieved at 33 s after contrast administration using the test bolus algorithm (purple arrow), with a 5 s time difference (red arrow) between the conventional technique and the test bolus algorithm. CCTA, coronary computed tomography angiography.
In order to optimize the scan delay for each patient, the software first predicts the expected contrast enhancement in the ascending aorta before the CCTA scan. In general, the algorithm considers a patient as a linear time-invariant (LTI) system so that the contrast enhancement over time, , can be described as a convolution of the injection protocol, IP(t), with the arterial impulse response (AIR) of the patient :
with in Hounsfield Units (HU), in mL/s, and in HU/mL. For a given , a patient-specific contrast enhancement can be predicted for any desirable injection protocol . First, the algorithm extracts the right function from the test bolus injection protocol and the test bolus signal ( ) in the descending aorta. Then, the algorithm predicts the contrast enhancement of the CCTA scan in the descending aorta on the basis of the previously derived and the injection protocol used for the CCTA scan. Finally, the software simply estimates the scan delay as the time to peak of the curve. In both the control and the study cohort, the protocol ensured that there was enough time for table adjustments and breath-hold instructions prior to image acquisition to ensure scanning at the optimal enhancement.
Objective image quality analysis
The CCTA data were analyzed on a dedicated workstation (syngo.via MM Reading, v. VB20A, Siemens) by a radiologist with 4 years of CCTA experience. Measurements included mean attenuation (HU) of the major coronary arteries (right, left main, left anterior descending, and left circumflex), the left and right ventricles, the ascending aorta, and the superior vena cava. Additional measurements were performed in the interventricular septum and mediastinal fat to assess image contrast and noise. Measurements were performed twice and averaged to ensure data consistency and high measurement accuracy. The following formulas were used for calculating signal-to-noise (SNR) and contrast-to-noise ratios (CNR):
SNR = HUartery/SDfat
CNR = (HUartery − HUseptum) / SDfat
Subjective image quality analysis
All CCTA scans were interpreted by two board certified cardiovascular radiologists. All images were independently reviewed, and the reviewers were blinded to the test bolus technique. To reduce recall bias, all image series were evaluated in random order. Subjective image quality of the coronary arteries was evaluated using a 5-point Likert scale ranging from 1 = poor image quality to 5 = excellent image quality.
Statistical analysis
Statistical analyses were performed using MedCalc v. 19.2 (MedCalc Software bvba, Ostend, Belgium) and R v. 4.1.0 (https://www.r-project.org/). Categorical variables were reported as frequency with percentage. Normal distribution was assessed using the Shapiro-Wilk test. Data are presented as mean ± standard deviation (SD) or as median with interquartile range (IQR). Group comparison was performed using the Mann-Whitney U-test and Fisher’s exact test/chi-square tests for continuous and categorical variables, respectively. To assess interobserver agreement, intraclass correlation coefficients (ICC) were calculated and interpreted as:<0.50, poor agreement; 0.50–0.75, moderate agreement; 0.75–0.90, good agreement; and >0.90, excellent agreement. Statistical significance was assumed at a p-value of <0.05.
Results
Study population
From a total of 36 patients, two were excluded due to renal dysfunction, one due to respiratory impairment and one due to unstable clinical status. Thus, the final study population comprised a total of 32 patients. The study population included 16 men (50%) and 16 women (50%) with a mean age of 59.4 ± 17.4 years. Patients in the control group were matched based on age, sex, body mass index (BMI) and tube voltage with a mean age of 59.4 ± 14 years. The two study cohorts did not significantly differ regarding age (p = 0.56), sex (p = 1)and BMI (p = 0.66). In addition, cardiac risk factors did not significantly differ between the cohorts. The mean CTDIvol and DLP in the study and control groups were 40.9 ± 30.7 mGy vs 42.9 ± 31.9 mGy, and 660.8 ± 575.6 mGy*cm vs 705.2 ± 373.2 mGy*cm, respectively. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Variables | Study group | Control group | p-value |
|---|---|---|---|
| N | 32 | 32 | |
| Age (years) | 59.4 ± 17.4 | 59.4 ± 14 | 0.563 |
| Gender (male) | 16 (50.0%) | 16 (50.0%) | 1 |
| BMI (kg/m2) | 29.2 ± 5.9 | 29.8 ± 5.6 | 0.656 |
| BMI ≥ 30 kg/m2 | 12 (37.5%) | 14 (43.8%) | 0.248 |
| Heart rate (bpm) | 68.5 ± 12.2 | 66.1 ± 10.7 | 0.318 |
| CTDI (mGy) | 40.9 ± 30.7 | 42.9 ± 31.9 | 0.783 |
| DLP (mGy*cm) | 660.8 ± 575.6 | 705.2 ± 373.2 | 0.763 |
| Tube voltage (kV) | 105.3 ± 21.9 | 106.9 ± 18.4 | 0.995 |
| Contrast media (ml) | 55.6 ± 13.3 | 55.8 ± 11.5 | 0.952 |
BMI, body mass index; CAD, coronary artery disease; CTDI, computed tomography dose index; DLP, dose length product.
Time delay for CCTA
The time to peak measured during test bolus administration was not different between the study group (19.2 ± 2.9 s) and the control group (19.1 ± 3.5 s; p = 0.95). In the study group, the mean time delay for CCTA determined by the prototype algorithm was significantly longer (26.0 ± 2.9 s; range 18.9–31.2 s) than that of the control group using the standard +4 s delay adjustment (23.1 ± 3.5 s; range 16.0–28.1 s; p < 0.01).
Objective image quality analysis
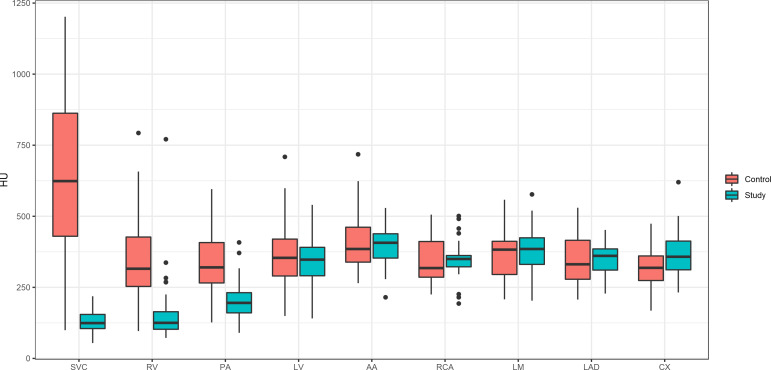
Mean attenuation, SNR and CNR values are reported in Table 2 and Figure 2. Attenuation values in the superior vena cava (128.7 HU vs 657.7 HU, p < 0.001), the right ventricle (142.8 HU vs 360 HU, p < 0.001), and the pulmonary artery (200.9 HU vs 334.8 HU, p < 0.001) and were significantly lower in the study group. The study cohort showed higher SNR in the RCA (17.5 vs 13.0; p = 0.028), the LM (15.3 vs 12.3; p = 0.027), and the LAD (18.5 vs 14.1; p = 0.048). The LCX showed no significant difference regarding SNR (19.6 vs 17.8; p = 0.525). In the study group, CNR was lower in the superior vena cava, the right ventricle and the pulmonary artery but not significantly different in the coronary arteries.
Table 2.
Objective image quality including attenuation (HU), SNR and CNR values
| Location | Attenuation (HU) | SNR | CNR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study group | Control group | p-value | Study group | Control group | p-value | Study group | Control group | p-value | |
| Superior vena cava | 128.7 ± 38.1 | 657.7 ± 290 | <0.001 | 4.4 ± 1.5 | 6.6 ± 5.6 | 0.036 | 1 ± 1.4 | 18.3 ± 9.4 | <0.001 |
| Right ventricle | 142.8 ± 128.7 | 360 ± 162.4 | <0.001 | 4.7 ± 1.8 | 11.4 ± 5.2 | <0.001 | 1.8 ± 3.0 | 8.8 ± 6.5 | <0.001 |
| Pulmonary artery | 200.9 ± 72.4 | 334.8 ± 102.7 | <0.001 | 7.8 ± 3 | 12.3 ± 5 | <0.001 | 3.7 ± 2.6 | 7.7 ± 3.9 | <0.001 |
| Left ventricle | 345.4 ± 89.9 | 362.2 ± 116.6 | 0.707 | 10.7 ± 3.2 | 10.9 ± 4.9 | 0.758 | 9.8 ± 4.8 | 9.4 ± 5.3 | 0.728 |
| Ascending aorta | 397.8 ± 67.2 | 410.3 ± 108.3 | 0.799 | 15.0 ± 5.3 | 15.0 ± 5.2 | 0.931 | 12.1 ± 5.1 | 10.8 ± 4.9 | 0.289 |
| Right coronary artery | 348.4 ± 67.9 | 348.9 ± 87.1 | 0.804 | 17.5 ± 10.3 | 13 ± 5.3 | 0.028 | 10.1 ± 4.7 | 8.4 ± 3.7 | 0.119 |
| Left main | 382.2 ± 76.1 | 361.6 ± 77.6 | 0.280 | 15.3 ± 5.3 | 12.3 ± 5.1 | 0.027 | 11.4 ± 4.9 | 9.3 ± 5.5 | 0.127 |
| Left anterior descending | 350.7 ± 49.8 | 356.7 ± 93.1 | 0.867 | 18.5 ± 9.9 | 14.1 ± 7 | 0.048 | 10.1 ± 4.2 | 8.6 ± 3.6 | 0.137 |
| Left circumflex | 365 ± 79.3 | 319 ± 81.1 | 0.036 | 19.6 ± 12.3 | 17.8 ± 10 | 0.525 | 10.5 ± 4.4 | 7.7 ± 4.2 | 0.011 |
CNR, contrast to noise ratio; HU, Hounsfield unit; SNR, signal to noise ratio.
Figure 2.
Box plot diagram shows opacification (HU) values at the different anatomic structures in the study group (blue) and the control group (orange). Significant differences can be observed in the SVC (superior vena cava), RV (right ventricle) and PA (pulmonary artery). LV, left ventricle; AA, ascending aorta; RCA, right coronary artery; LM, left main; LAD, left anterior descending; LCX, left circumflex artery.
Subjective image quality analysis
Subjective image quality ratings were significantly higher in the study group compared to the control group (4.75 ± 0.7 vs 3.64 ± 0.5; p < 0.001). In Figure 3, corresponding images of both the study group and the control group are represented. Interobserver agreement was good for both the study group (ICC 0.86) and the control group (ICC 0.79).
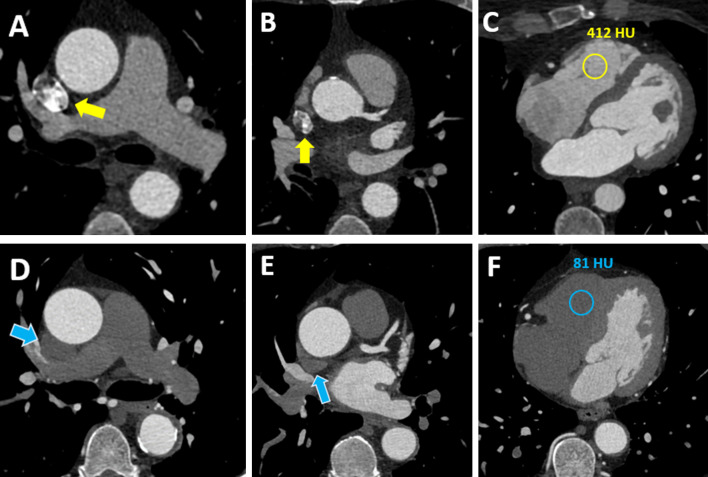
Figure 3.
66 year-old female with chest pain underwent standard CCTA (Case 1, (A-C) and 65-year-old male with known coronary artery disease and recurrence of angina underwent CCTA using the test bolus evaluation algorithm (Case 2, (D-F). Axial CCTA sections are shown. Beam hardening artifact is shown in the superior vena cava in case one with standard CCTA (A, B; yellow arrows), while artifact is not present in Case two with test bolus algorithm based CCTA (D, E; blue arrows). Furthermore, panel (C) shows a contrast filled right ventricle with a density of 412 HU. In contrast, panel (F) demonstrates low attenuation in the right ventricle (81 HU). CCTA, coronary computed tomography angiography; HU = Hounsfield Units.
Discussion
In this study, we prospectively investigated a test bolus evaluation prototype algorithm in patients undergoing CCTA with the aim of optimizing individual acquisition timing after CM administration to ensure homogeneous contrast attenuation in the coronary arteries. Our data suggest that circulation time-based patient-specific timing adjustment provides additional value by optimizing CM administration and scan delay for CCTA examinations, thus improving image quality. None of the patients who underwent CCTA with an individualized trigger delay showed non-diagnostic image quality.
An optimal contrast protocol should guarantee uniform, prolonged contrast attenuation during the CCTA scan. Using these techniques, we were able to show stable contrast enhancement between 300 and 400 HU in the coronary arteries. This is of particular importance for CCTA because optimal contrast attenuation in the coronary arteries has a considerable influence on image quality and the ability to evaluate images for stenosis and arteriosclerotic changes. 5,13 In addition to sufficient contrast attenuation above 200 HU, which allows adequate evaluation of smaller vessels, it should also be noted that attenuation beyond 400 HU may limit the evaluation of coronary calcifications. 5,14 In contrast to the control group, the individually timed and uniform CM application avoids the presence of beam hardening artifacts caused by iodinated CM e.g. in the superior vena cava, which otherwise would have negative impact on image interpretation. 15–17
The mean and peak level of arterial contrast enhancement show substantial interindividual differences, even when CM is administered at the same volume and flow rate. These differences are confirmed by the wide range of time delay to peak attenuation observed in our study cohort. Several physiologic parameters including body weight, body surface area, central blood volume and circulation time all influence arterial peak enhancement. In fact, circulation time proves to be the physiologic factor with the greatest impact on the magnitude and timing of contrast enhancement. 14,18–21 Therefore, patients with pre-existing cardiac conditions or impaired cardiac function stand to benefit the most from individualized contrast media delay timing which factors in the individuals’ circulation time.
One of the most important methods currently used is test bolus injection. 22 Sandfort et al examined a total of 151 patients using three different test bolus protocols. 23 The authors investigated the enhancement of the ascending aorta and showed that a protocol, which included a lower flow rate but a higher concentration of contrast agent, demonstrated a lower and more stable range of attenuation values compared to the other two protocols. The remaining protocols tested were a standard test bolus protocol and a body weight adapted protocol which showed the highest standard deviation of aortic enhancement. 23,24 However, all protocols tested considered the maximum arterial enhancement as the delay, which factors in each individual’s CO. Seifarth et al also investigated three different test bolus protocols in 120 patients using different amounts of contrast agent and flow rates. In addition to the previous study these three protocols were based on the peak enhancement curve of the ascending aorta +4 s. However, this calculation is only an approximation to the individual circulation time. Exact measurement methods of cardiac output and circulation time include echocardiography, angiocardiography, thermo-dilution by Swan-Ganz catheter and advanced imaging methods of cardiac magnetic resonance imaging and CCTA. 25,26 These cardiac output measurements are rarely used in everyday clinical practice to determine the time delay to maximum enhancement in the ascending aorta before CCTA. Whereas CCTA-based estimation of circulation time would only be available after the completion of the imaging study.
In order to take into account, the myriad of influences on circulation time and the associated variability of individual flow velocity, we tested a test-bolus algorithm, which calculated the interindividual delay based on the patient-specific flow curve of the test-bolus injection protocol by taking into account a substantial, extensive database of archived improvement curves for its precise calculations. Embedding this approach into a routine workflow for a CT scan, we ensured that by determining the individual delay time, a sufficient and homogeneous contrast within the desired scan area could be provided in order to obtain noticeably better objective and subjective image quality compared to that of the control group.
To our knowledge, there are no comparable studies that have used a similar test-bolus algorithm for CCTA. In a prospective study, Hinzpeter et al examined 108 participants with CTA of the aorta by comparing a cohort with fixed trigger delay and a cohort with patient-specific, individualized trigger delay for contrast medium timing. 27 It was shown that patients with individualized trigger delay were accompanied by more uniform contrast attenuation and improved image quality. Instead of a test bolus algorithm, they used the bolus tracking method for patient-specific trigger delays.
There are several study limitations that need to be considered. First, this is a single-center study with a relatively small cohort size. Second, cohort assignment was not randomized. Our study focused solely on CCTA and its objective and subjective image quality. The extent to which other arterial or pulmonary arterial, patient-specific time delays would be proven by the test bolus evaluation was outside the scope of this study. Moreover, the variations of the patients’ specific circulation time may have influenced our study results. The test-bolus evaluation was associated with a slightly longer time required to transfer data to an external computer for calculation, which should improve with evolving clinical integration. Additionally, in this study we only adjusted the time delay and did not alter other variables such as test bolus volume and flow. Further analyses on this patient specific approach are needed to provide an individually tailored CCTA protocol.
Conclusion
In conclusion, the patient-specific test bolus evaluation algorithm for CCTA reliably provided a patient specific scan delay to ensure constant vascular attenuation, which led to an improvement in objective and subjective image quality in addition to the avoidance of beam hardening artifacts. This technique has the potential to benefit patients with decreased circulation time who present for CCTA exams by individualizing scan parameters to the benefit of each patient.
Footnotes
Funding: This study was supported by Siemens Healthcare.
Disclosure: Akos Varga-Szemes receives institutional research and travel support from Siemens Healthcare and is a consultant for Bayer and Elucid Bioimaging. U. Joseph Schoepf is a consultant for and / or receives research support from Bayer, Bracco, Elucid Bioimaging, Guerbet, HeartFlow, and Siemens Healthcare. Thomas Flohr, Bernhard Schmidt, Ralf Gutjahr, and Pooyan Sahbaee are employees of Siemens Healthcare. Tilman Emrich receives travel support and speaker fee from Siemens Healthcare.
The authors Andreas M Fischer and Josua A. Decker contributed equally to the work.
Contributor Information
Andreas M Fischer, Email: andreasmarco.fischer@googlemail.com, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA ; University Department of Geriatric Medicine FELIX PLATTER and University of Basel, Basel, Switzerland .
Josua A. Decker, Email: josua.decker@uk-augsburg.de, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA Department of Diagnostic and Interventional Radiology, University Hospital Augsburg, Augsburg, Germany .
Joseph Schoepf, Email: schoepf@musc.edu, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA .
Akos Varga-Szemes, Email: vargaasz@musc.edu, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA .
Thomas Flohr, Email: thomas.flohr@siemens-healthineers.com, Siemens Healthcare, Forchheim, Germany .
Bernhard Schmidt, Email: bernhard.schmidt@siemens-healthineers.com, Siemens Healthcare, Forchheim, Germany .
Ralf Gutjahr, Email: ralf.gutjahr@siemens-healthineers.com, Siemens Healthcare, Forchheim, Germany .
Pooyan Sahbaee, Email: pooyan.sahbaee@siemens-healthineers.com, Siemens Medical Solutions, Ann Arbor, Michigan, USA .
Dante A Giovagnoli, Email: giovagnd@musc.edu, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA .
Tilman Emrich, Email: emrich@musc.edu, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA ; Department of Diagnostic and Interventional Radiology, University Medical Center, Mainz, Germany ; German Center for Cardiovascular Research (DZHK), Partner Site Rhine Main, Mainz, Germany .
John D Martinez, Email: martjohn@musc.edu, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA .
Kia B Lari, Email: klari@email.sc.edu, University of South Carolina School of Medicine Greenville, Greenville, South Carolina, USA .
Robert R Bayer, II, Email: bayer@musc.edu, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA ; Division of Cardiology, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, USA .
Simon S Martin, Email: simartin@outlook.com, Division of Cardiovascular Imaging, Department of Radiology and Radiological Science, Medical University of South Carolina, Charleston, South Carolina, USA ; Department of Diagnostic and Interventional Radiology, University Hospital Frankfurt, Frankfurt, Germany .
REFERENCES
- 1. Schoepf UJ, Zwerner PL, Savino G, Herzog C, Kerl JM, et al. . Coronary ct angiography . Radiology 2007. ; 244: 48 – 63 . doi: 10.1148/radiol.2441052145 [DOI] [PubMed] [Google Scholar]
- 2. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, et al. . Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter accuracy (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial . J Am Coll Cardiol 2008. ; 52: 1724 – 32 . doi: 10.1016/j.jacc.2008.07.031 [DOI] [PubMed] [Google Scholar]
- 3. Maroules CD, Rajiah P, Bhasin M, Abbara S . Current evidence in cardiothoracic imaging: growing evidence for coronary computed tomography angiography as a first-line test in stable chest pain . J Thorac Imaging 2019. ; 34: 4 – 11 . doi: 10.1097/RTI.0000000000000357 [DOI] [PubMed] [Google Scholar]
- 4. Johnson TRC, Nikolaou K, Wintersperger BJ, Leber AW, von Ziegler F, et al. . Dual-source ct cardiac imaging: initial experience . Eur Radiol 2006. ; 16: 1409 – 15 . doi: 10.1007/s00330-006-0298-y [DOI] [PubMed] [Google Scholar]
- 5. Mahnken AH, Rauscher A, Klotz E, Mühlenbruch G, Das M, et al. . Quantitative prediction of contrast enhancement from test bolus data in cardiac msct . Eur Radiol 2007. ; 17: 1310 – 19 . doi: 10.1007/s00330-006-0486-9 [DOI] [PubMed] [Google Scholar]
- 6. Eberhard M, Alkadhi H . Machine learning and deep neural networks: applications in patient and scan preparation, contrast medium, and radiation dose optimization . J Thorac Imaging 2020. ; 35 Suppl 1: S17 – 20 . doi: 10.1097/RTI.0000000000000482 [DOI] [PubMed] [Google Scholar]
- 7. Konno M, Hosokai Y, Usui A, Abe M, Tateishi T, et al. . Cardiac output obtained from test bolus injections as a factor in contrast injection rate revision of following coronary ct angiography . Acta Radiol 2012. ; 53: 1107 – 11 . doi: 10.1258/ar.2012.120276 [DOI] [PubMed] [Google Scholar]
- 8. Ichikawa S, Yamamoto H, Ito O, Fukunaga M . Pulmonary artery/vein separation using single-phase computed tomography: feasibility and the influence of patient characteristics on vessel enhancement . J Thorac Imaging 2020. ; 35: 173 – 78 . doi: 10.1097/RTI.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 9. Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, et al. . SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: endorsed by the north american society for cardiovascular imaging (nasci) . J Cardiovasc Comput Tomogr 2016. ; 10: 435 – 49 . doi: 10.1016/j.jcct.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 10. Sahbaee P, Segars WP, Marin D, Nelson RC, Samei E . The effect of contrast material on radiation dose at ct: part i . Incorporation of Contrast Material Dynamics in Anthropomorphic Phantoms Radiology 2017. ; 283: 739 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sahbaee P, Abadi E, Segars WP, Marin D, Nelson RC, et al. . The effect of contrast material on radiation dose at ct: part ii. a systematic evaluation across 58 patient models . Radiology 2017. ; 283: 749 – 57 . doi: 10.1148/radiol.2017152852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahbaee P, Segars PP, Marin D, Nelson R, Samei E . Determination of contrast media administration to achieve a targeted contrast enhancement in computed tomography . J Med Imaging (Bellingham) 2016. ; 3: 013501 . doi: 10.1117/1.JMI.3.1.013501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker CR, Hong C, Knez A, Leber A, Bruening R, et al. . Optimal contrast application for cardiac 4-detector-row computed tomography . Invest Radiol 2003. ; 38: 690 – 94 . doi: 10.1097/01.rli.0000084886.44676.e4 [DOI] [PubMed] [Google Scholar]
- 14. Husmann L, Alkadhi H, Boehm T, Leschka S, Schepis T, et al. . Influence of cardiac hemodynamic parameters on coronary artery opacification with 64-slice computed tomography . Eur Radiol 2006. ; 16: 1111 – 16 . doi: 10.1007/s00330-005-0110-4 [DOI] [PubMed] [Google Scholar]
- 15. Bucher AM, Wichmann JL, Schoepf UJ, Wolla CD, Canstein C, et al. . Quantitative evaluation of beam-hardening artefact correction in dual-energy ct myocardial perfusion imaging . Eur Radiol 2016. ; 26: 3215 – 22 . doi: 10.1007/s00330-015-4137-x [DOI] [PubMed] [Google Scholar]
- 16. So A, Hsieh J, Li JY, Lee TY . Beam hardening correction in ct myocardial perfusion measurement . Phys Med Biol 2009. ; 54: 3031 – 50 . doi: 10.1088/0031-9155/54/10/005 [DOI] [PubMed] [Google Scholar]
- 17. Henry TS, Hammer MM, Little BP, Jensen LE, Kligerman SJ, et al. . Smoke: how to differentiate flow-related artifacts from pathology on thoracic computed tomographic angiography . J Thorac Imaging 2019. ; 34: W109 – 20 . doi: 10.1097/RTI.0000000000000429 [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi T . Scanning techniques of ccta that uses 64-rows mdct . Nihon Hoshasen Gijutsu Gakkai Zasshi 2009. ; 65: 104 – 11 . doi: 10.6009/jjrt.65.104 [DOI] [PubMed] [Google Scholar]
- 19. Sakai S, Yabuuchi H, Chishaki A, Okafuji T, Matsuo Y, et al. . Effect of cardiac function on aortic peak time and peak enhancement during coronary ct angiography . Eur J Radiol 2010. ; 75: 173 – 77 . doi: 10.1016/j.ejrad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 20. Bae KT . Intravenous contrast medium administration and scan timing at ct: considerations and approaches . Radiology 2010. ; 256: 32 – 61 . doi: 10.1148/radiol.10090908 [DOI] [PubMed] [Google Scholar]
- 21. Mahnken AH, Klotz E, Hennemuth A, Jung B, Koos R, et al. . Measurement of cardiac output from a test-bolus injection in multislice computed tomography . Eur Radiol 2003. ; 13: 2498 – 2504 . doi: 10.1007/s00330-003-2054-x [DOI] [PubMed] [Google Scholar]
- 22. Sun K, Liu G-R, Li Y-C, Han R-J, Cui L-F, et al. . Intravenous contrast material administration at high-pitch dual-source ct coronary angiography: bolus-tracking technique with shortened time of respiratory instruction versus test bolus technique . Chin Med Sci J 2013. ; 27: 225 – 31 . doi: 10.1016/s1001-9294(13)60006-1 [DOI] [PubMed] [Google Scholar]
- 23. Sandfort V, Choi Y, Symons R, Chen MY, Bluemke DA . An optimized test bolus contrast injection protocol for consistent coronary artery luminal enhancement for coronary ct angiography . Acad Radiol 2020. ; 27: 371 – 80 . doi: 10.1016/j.acra.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Yu M, Wang M, Wang Y, Kong L, et al. . Application of artificial intelligence-based image optimization for computed tomography angiography of the aorta with low tube voltage and reduced contrast medium volume . J Thorac Imaging 2019. ; 34: 393 – 99 . doi: 10.1097/RTI.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 25. Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, et al. . Minimally invasive or noninvasive cardiac output measurement: an update . J Anesth 2016. ; 30: 461 – 80 . doi: 10.1007/s00540-016-2154-9 [DOI] [PubMed] [Google Scholar]
- 26. Hofer CK, Ganter MT, Zollinger A . What technique should i use to measure cardiac output? Curr Opin Crit Care 2007. ; 13: 308 – 17 . doi: 10.1097/MCC.0b013e3280c56afb [DOI] [PubMed] [Google Scholar]
- 27. Hinzpeter R, Eberhard M, Gutjahr R, Reeve K, Pfammatter T, et al. . CT angiography of the aorta: contrast timing by using a fixed versus a patient-specific trigger delay . Radiology 2019. ; 291: 531 – 38 . doi: 10.1148/radiol.2019182223 [DOI] [PubMed] [Google Scholar]