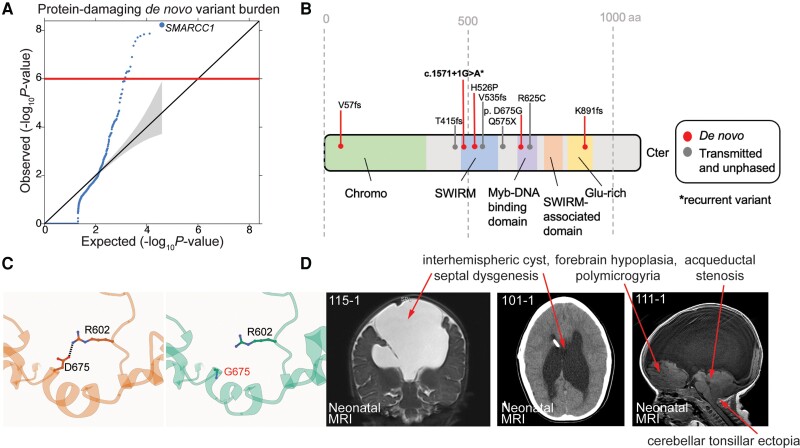
Figure 1.
SMARCC1 mutations are associated with congenital hydrocephalus and cause a novel human BAFopathy featuring cerebral ventriculomegaly. (A) Quantile–quantile (Q-Q) plot of observed versus expected P-values for de novo variants (DNVs) in each gene in 2697 trio cases. P-values were calculated using a one-sided Poisson test (refer to the ‘Materials and methods’ section). For protein-damaging de novo SMARCC1 variants [loss-of-function (LoF), MetaSVM = D and/or MPC > 2], P = 5.83 × 10−9. (B) Schematic diagram showing variant locations in SMARCC1 protein domains. Identified DNVs, transmitted and unknown inheritance variants15,16 are indicated with markers. *Recurrent variant. (C) The p.Asp675Gly variant was predicted to be detrimental to SMARCC1 structure and function by alpha-fold biophysical modelling. Structural protein modelling predicts that p.Asp675Gly alters a conserved residue in the Myb domain resulting in loss of an ion pair interaction with p.Arg602, with a predicted ΔG of 0.73 kcal/mol. (D) Brain MRIs of congenital hydrocephalus patients with SMARCC1 variants demonstrate consistent structural abnormalities. Prenatal imaging is shown for Patients 115-1 (contrast MRI), 101-1 and 111-1. Red asterisks denote ventricular catheter of a ventriculo-peritoneal shunt used to treat obstructive hydrocephalus.