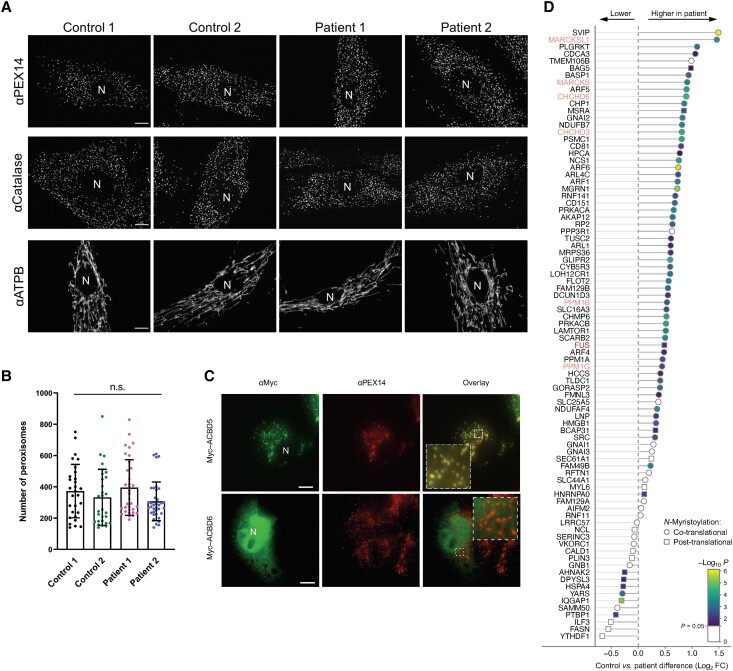
Figure 6.
Morphological characteristics of peroxisomes in ACBD6-deficient patient cells are not altered and chemical proteomic profiling of N-myristoylation in human fibroblasts. (A) Patient fibroblasts and controls were processed for immunofluorescence microscopy using antibodies against the peroxisomal membrane marker PEX14, the matrix marker catalase or mitochondrial ATP synthetase B (ATPB). Peroxisomal localization of PEX14 and catalase indicate that peroxisomal membrane and PTS1-dependent matrix import are normal. Note that the morphology of mitochondria, which are elongated in fibroblasts, was also not altered when compared to controls. (B) Quantification of peroxisome number based on immunofluorescence images (see A for representative images) (n = 29–36 cells). Data are from three independent experiments. ns, not significant; Kruskal–Wallis ANOVA test with Dunn’s multiple comparisons. (C) COS-7 cells were transfected with plasmids encoding Myc-ACBD5 or Myc-ACBD6 and processed for immunofluorescence microscopy using antibodies against Myc and PEX14. Note that Myc-ACBD5 localizes to peroxisomes, whereas Myc-ACBD6 localizes to the nucleus and the cytoplasm in COS-7 cells. Scale bars = 10 µm. (D) Ranked plot of myristic acid alkyne (YnMyr)-labelled, known co- and post-translationally N-myristoylated proteins, as identified in Supplementary Fig. 18E. Position on the left equals lower abundance in ACBD6 deficient fibroblasts, position on the right equals higher abundance in ACBD6 deficient fibroblasts.