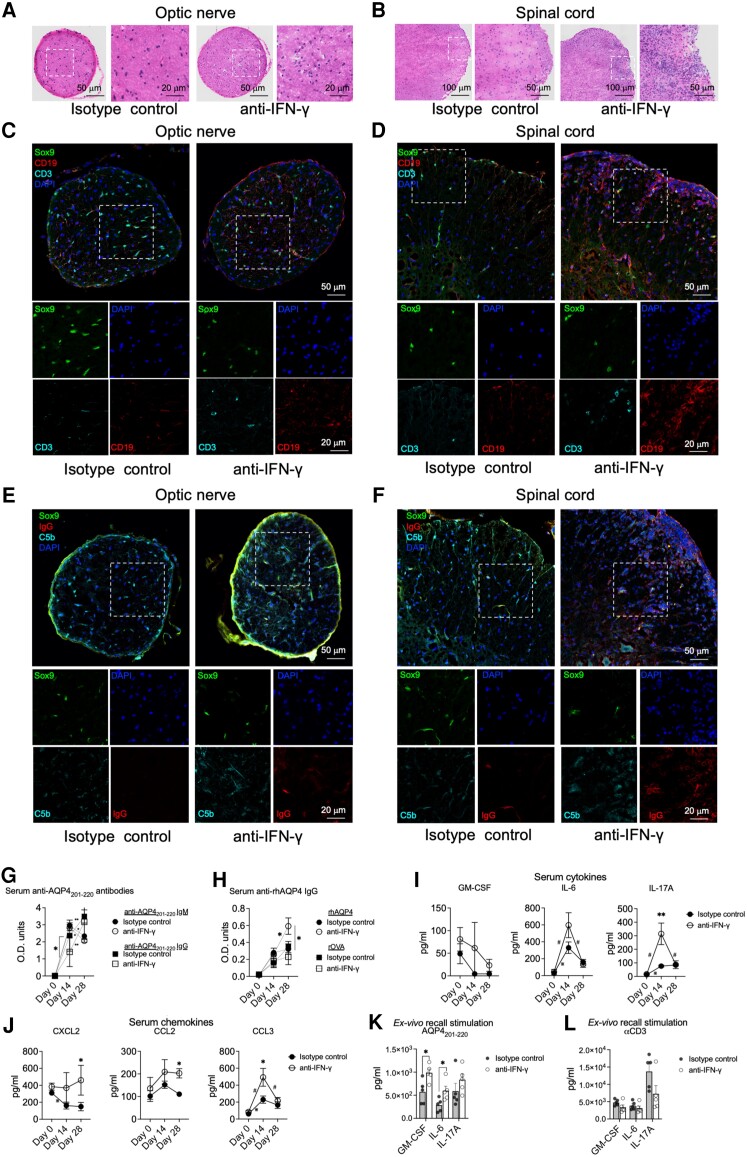
Figure 3.
IFN-γ regulates peripheral immune responses and CNS infiltration of inflammatory cells in AQP4201–220-induced disease. Optic nerve (A, C and E) and spinal cord (B, D and F) sections from wild-type (WT) mice treated with either anti-IFN-γ or isotype control antibodies were analysed at Day 22 post-immunization (p.i.) using haematoxylin and eosin staining (A and B), and Sox9-CD3-CD19-DAPI (C and D), Sox9-C5b-IgG-DAPI (E and F) immunostaining. Scale bars for 20, 50 and 100 μm are indicated on the images. Optical densities of total serum levels of anti-AQP4201–220-specific IgM (circles) and IgG (squares) in peptide-immunized mice treated with anti-IFN-γ (open circles, n = 9) or isotype control (filled circles, n = 11) antibodies were determined by ELISA before immunization at Day 0 and at Days 14 and 28 p.i. (G). Serum IgG antibody levels against recombinant human AQP4 (rhAQP4) or rOVA (control protein) were also determined (H). Serum levels of GM-CSF, IL-6 and IL-17 cytokines (I), and CXCL2, CCL2 and CCL3 chemokines (J) from AQP4201–220-immunized mice treated with anti-IFN-γ (n = 6) or isotype control (n = 6) antibodies were determined by multiplex cytokine assay prior immunization at Day 0 and at Days 14 and 28 p.i. Ex vivo recall analysis was performed using supernatants (multiplex cytokine analysis of GM-CSF, IL-6 and IL-17A) from splenocytes of anti-IFN-γ-treated (n = 5) or isotype control-treated (n = 5) groups obtained at Day 18 p.i. and following cell culture for 72 h in the presence of 40 μg/ml AQP4201–220 peptide (K) or 1 μg/ml of soluble anti-CD3 antibody (L). All data are presented as mean ± SEM. Statistical analyses were performed using the Mann–Whitney test: *P < 0.05, **P < 0.01; #,*statistically significant difference between Days 0–14 or 14–28 p.i. in anti-IFN-γ- or isotype control-treated groups, respectively.