Abstract
Objective: To investigate the efficacy of human immunoglobulin combined with antibiotics in treating severe pediatric pneumonia. Methods: A retrospective analysis was performed on 210 pediatric patients with severe pneumonia admitted to the Department of Neonatology of Cangzhou Central Hospital from April 2019 to October 2022. Patients were divided into two groups (the observation group and the control group) based on the administration of human immunoglobulin. Clinical indexes of both groups before and after treatment were analyzed to determine the therapeutic effect of different treatment methods on pediatric severe pneumonia. Results: The durations of cough, fever, pulmonary rales, and lung shadow, and hospitalization time in the observation group were significantly shorter than those in the control group (all P<0.05). The total clinical effective rate in the observation group was significantly higher than that in the control group (P<0.05). Levels of inflammatory factors (IL-6, IL-8 and hsCRP) were decreased in both groups after treatment (all P<0.05), and were lower in the observation group compared with the control group after treatment (all P<0.05). The serum levels of IgA, IgG and IgM after five days of intervention were obviously higher than those before intervention in the observation group (all P<0.05), but the serum levels of IL-4, INF-γ and INF-γ/IL-4 were obviously lower (all P<0.05). The total incidence of adverse reactions between two groups after intervention was not statistically different (P<0.05). Conclusion: The combination of human immunoglobulin and antibiotics for the treatment of pediatric severe pneumonia is beneficial, because it improves efficacy, boosts the immune system, and reduces inflammation.
Keywords: Severe pneumonia, human immunoglobulin, immune function, inflammatory response
Introduction
Severe pneumonia is a specific type of pneumonia with a persistent and widespread disease burden [1,2]. Children with severe pneumonia develop high fever, moderate to severe systemic symptoms, and an increased risk of damage to other organs. Mycoplasma pneumonia is caused by Mycoplasma, a common bacterial pathogen associated with a variety of clinical manifestations (upper respiratory tract infection and pneumonia). Mycoplasma pneumonia often occurs in the community, mostly in autumn and winter, with sporadic cases in other seasons, especially among school-age children and young adults [3,4]. Mycoplasma pneumoniae pneumonia (MPP) is the most common community-acquired pneumonia (CAP) in children aged 5 or older in China [5]. Early detection of severe and critical cases, rational treatment, and avoidance of death and sequelae are the core and key issues in the management of MPP.
The pathogenesis of MPP has not been fully elucidated, but there are currently two main mechanisms [6,7]: direct damage by MP and abnormal immune response of the host. Abnormal host immune responses to MP infection can lead to immune damage in the lung and extrapulmonary tissues through multiple pathways, including autoimmune reactions, allergic reactions, and immune complex formation. Abnormal host immune responses play an important role in the development of SMPP, FMPP and extrapulmonary complications and lead to clinical and imaging diversity of MPP [8].
In clinical practice, the treatment of pediatric severe pneumonia is complex and conventional antibiotic therapy is not effective. The occurrence of severe pneumonia is associated with factors such as susceptibility, immune insufficiency, and weak resistance [9-12]. Immunoglobulins are immune agents extracted from plasma that, when administered intravenously, neutralize pathogenic bacteria and modulate the immune and inflammatory response in patients with infectious diseases [13,14].
Immunoglobulin combined with antimicrobial agents for the treatment of severe mycoplasma pneumonia has been studied more frequently [15,16]. In order to clarify the value of human immunoglobulin in the treatment of severe mycoplasma pneumonia, this study specifically analyzed the efficacy of human immunoglobulin combined with antibiotics in the treatment of severe mycoplasma pneumonia in pediatric patients and its effects on immune function and inflammatory response.
Materials and methods
Case selection
Data of 210 children diagnosed with severe mycoplasma pneumonia treated in Cangzhou Central Hospital from April 2019 to October 2022 were retrospectively selected and grouped into two groups according to the treatment regimen. A total of 105 patients treated with azithromycin alone were assigned to the control group, and the other 105 patients treated with human immunoglobulin combined with azithromycin were assigned to the observation group. This study was approved by the Medical Ethics Committee of Cangzhou Central Hospital.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients with persistent high fever for more than 5 days or fever for more than 7 days with no tendency of decreasing peak temperature. (2) Patients with wheezing, shortness of breath, dyspnea, chest pain and hemoptysis. These manifestations are associated with severe lesions, combined with plastic bronchitis, asthma attacks, pleural effusions and pulmonary embolism. (3) Patients who presented extra-pulmonary complications, but did not meet the criteria for critical illness. (4) Patients with finger pulse oxygen saturation less than 0.93 on air inhalation at rest. (5) Patients with any of following image manifestations: ① uniform-density mass lesions in more than 2/3 of a single lung lobe, or dense mass lesions in 2 or more lobes (regardless of the size of the area involved), which may be accompanied by moderate to massive pleural effusion or limited manifestations of fine bronchiectasis; ② diffuse single lung with manifestations of fine bronchiectasis, which may be combined with bronchiectasis and mucus plug formation leading to pulmonary atelectasis. (6) Progressive worsening of clinical symptoms, with imaging showing more than 50% progression of lesion extent within 24-48 h. (7) The guardians agreed to all treatment plans and signed the informed consent.
Exclusion criteria: (1) Children who were allergic to azithromycin or human immunoglobulin. (2) Children with a history of aspiration or aspiration pneumonia. (3) Children with immunocompromising or chronic medical conditions that predispose to severe or recurrent pneumonia (e.g., immunodeficiency, chronic corticosteroid use, chronic lung disease, malignancy, sickle cell disease, congenital heart disease, patients dependent on tracheostomy, and neuromuscular disorders impacting respiration). (4) Patients with incomplete medical records.
Treatment program
Children in both groups were treated with oxygen inhalation, cough suppressants and antipyretics, and the control group was given azithromycin anti-infection treatment: intravenous azithromycin administration (Shandong Luoxin Pharmaceutical Group Co., Ltd.; specification: 0.125 g × 1 bottle) 10 mg/(kg-d) for 5 days, followed by an equal amount of azithromycin oral solution (Shandong Luoxin Pharmaceutical Group Co., Ltd.; specification: 0.1 g × 4 sachets/times: 0.1 g × 4 bags/box) once per day for 5 consecutive days after the body temperature was normalized. In the observation group, human immunoglobulin (Hualan Bioengineering Pharmaceutical Co.; specification: 2.5 g (5%, 50 ml)) was added through intravenous drip, 1 time/d for 5 days.
Data collection
Demographic and clinical information, such as hs-C-reactive protein (hs-CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), immunoglobulin A (IgA) (Add& Read Human IgG Kit, Vazyme, China), immunoglobulin G (IgG) (Add& Read Human IgG Kit, Vazyme, China), immunoglobulin M (IgM) (IgM ELISA Kit, Abnova) and interferon gamma (IFN-γ) were retrospectively collected from all patients by reviewing their electronic medical records. During the hospitalization, adverse reactions and symptoms of patients were obtained, including nausea, vomit, rash, abdominal pain, diarrhea and so on.
The clinical efficacy of the two groups was compared. Markedly effective: After therapy, the chest CT scan showed that the lungs of the patient were normal; Effective: After therapy, the patient’s clinical symptoms were alleviated to a certain extent, and the chest CT scan results showed that the patient’s lungs had alleviated to a certain extent; Ineffective: After therapy, the patient’s clinical symptoms and chest CT scans results were not changed. Total effective treatment rate = [(number of cases with markedly effective + cases of effective)/the total number of patients] × 100%.
Detection of immune and inflammatory biomarkers
Three to five milliliters of venous blood were collected before and after treatment and the serum was centrifuged. The enzyme-linked immunosorbent assay (ELISA) was used to determine immunoglobulin A (IgA) (Add& Read Human IgG Kit, Vazyme, China), immunoglobulin G (IgG) (Add& Read Human IgG Kit, Vazyme, China), immunoglobulin M (IgM) (IgM ELISA Kit, Abnova), interferon gamma (IFN-γ) (Human IFN-γ ELISA Kit, Beyotime, China), interleukin-4 (IL-4) (Human IL-4 ELISA Kit, Beyotime, China), hypersensitive C-reactive protein (hs-CRP) (Human hs-CRP ELISA Kit, WELLBI, China), interleukin-6 (IL-6) (CSB-E04638h, CUSABIO, Wuhan, China) and interleukin-8 (IL-8) (CSB-E04641h, CUSABIO, Wuhan, China). All ELISA experiments were measured in a Thermo Fisher Microplate Reader.
Statistical analysis
SPSS 20.0 software (Chicago SPSS Co., Ltd.) was used for statistical analysis. Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as the number of cases and percentages (%). The independent t-test was used for comparison between the two groups, and the paired t-test was used for comparison of the same group at different time periods, and the results were expressed by t. The categorical variables were compared by chi-square test. P<0.05 indicated a significant difference.
Results
Basic data
Table 1 shows the characteristics of the patients in the two groups. There were 105 children in the control group with a mean age of 7.14±1.92 years. There were 105 children in the observation group with a mean age of 7.3±2.3 years. The two groups were comparable in terms of sex, age, course of disease, body temperature, BMI and clinical symptoms (all P>0.05).
Table 1.
Comparison of general information between the two groups
| Variables | Control (n=105) | Observation (n=105) | t/χ2 | p-value |
|---|---|---|---|---|
| Age | 7.1±1.9 | 7.3±2.3 | 1.312 | 0.322 |
| Gender | ||||
| Male | 45 (42.86%) | 50 (47.62%) | 0.308 | 0.579 |
| Female | 60 (57.14%) | 55 (52.38%) | ||
| Course of disease (days) | 8.84±1.77 | 8.39±1.67 | 0.134 | 0.066 |
| Body temperature | 39.66±0.42 | 39.22±0.32 | 0.342 | 0.087 |
| Body mass index | 18.87±0.22 | 18.46±0.32 | 0.214 | 0.079 |
| Clinical symptoms | ||||
| Fever | 100 (95.24%) | 103 (98.1%) | 0.312 | 0.096 |
| Dyspnea | 68 (64.76%) | 78 (74.29%) | 0.229 | 0.078 |
| Hypotension | 54 (51.43%) | 68 (64.76%) | 0.331 | 0.068 |
| Shock | 89 (84.76%) | 79 (74.24%) | 0.412 | 0.053 |
| Cough | 98 (93.33%) | 101 (96.19%) | 0.342 | 0.056 |
| Bellyache | 78 (74.29%) | 83 (79.05%) | 0.442 | 0.059 |
| Myocarditis | 59 (56.19%) | 54 (51.43%) | 0.487 | 0.055 |
Comparison of clinical therapeutic effect between the two groups
As shown in Table 2, the total effective rate in the observation group was 83.8% (88/105), which was significantly higher than the 63.8% in control group (P<0.05).
Table 2.
Comparison of clinical therapeutic effect between the two groups
| Therapeutic effect | Observation group (n=105) | Control group (n=105) | χ 2 | P |
|---|---|---|---|---|
| Significant effective | 34 (32.38%) | 19 (18.10%) | 7.368 | 0.008 |
| Effective | 54 (51.43%) | 46 (43.81%) | 9.837 | 0.022 |
| Ineffective | 11 (10.48%) | 34 (32.38%) | 4.161 | 0.013 |
| Total effective rate | 88 (83.81%) | 67 (63.81%) | 6.478 | 0.012 |
| t | 4.957 | 5.632 | - | - |
| P | 0.062 | 0.071 | - | - |
Comparison of serum levels of immune factors in observation group before and five days after intervention
The serum levels of IgA, IgG and IgM after five days of intervention were obviously higher than those before intervention in the observation group (all P<0.05). On the other hand, the serum levels of IL-4, INF-γ and INF-γ/IL-4 after five days of intervention were obviously lower than those before intervention in the observation group (all P<0.05, Figure 1).
Figure 1.
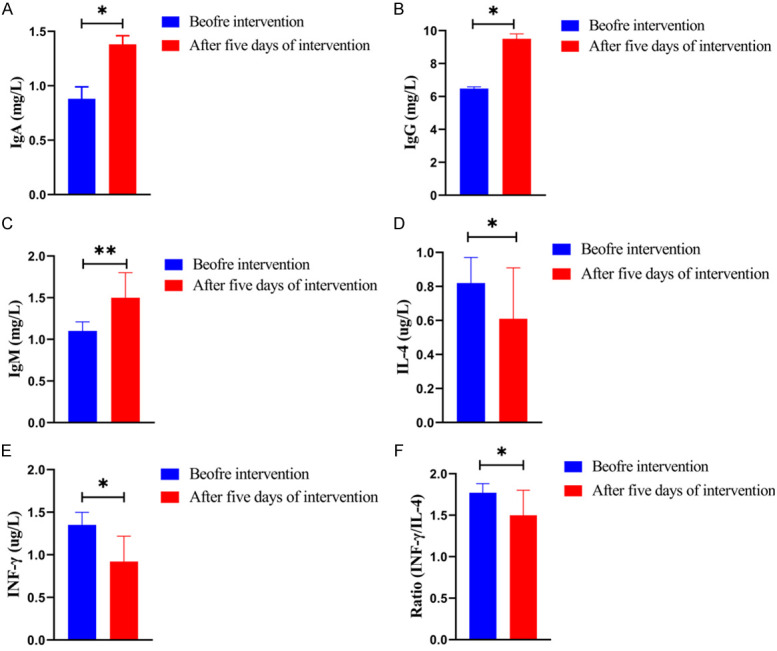
Comparison of immune biomarkers in the observation group before and five days after intervention. A: Comparison of serum levels of IgA before and after five days of intervention; B: Comparison of serum levels of IgG before and after five days of intervention; C: Comparison of serum levels of IgM before and after five days of intervention; D: Comparison of serum levels of IL-4 before and after five days of intervention; E: Comparison of serum levels of INF-γ before and after five days of intervention; F: Comparison of serum levels of INF-γ/IL-4 before and after five days of intervention; Note: Compared with before intervention, *P<0.05, **P<0.01. Note: IgA: Immunoglobulin A; IgG: Immunoglobulin G; IgM: Immunoglobulin M; IL-4: Interleukin-4; INF-γ: Interferon gamma.
Comparison of serum levels of inflammatory factors between the two groups
There was no significant difference in inflammatory factors (IL-6, IL-8 and hsCRP) between the two groups before treatment (P>0.05). After five days of intervention, the levels of above indicators were obviously decreased in both groups, and the levels in the observation group were significantly lower than those in the control group after intervention (all P<0.05, Figure 2).
Figure 2.

Comparison of Serum levels of inflammatory factors between the two groups before and five days after intervention. A: Comparison of serum levels of IL-6 between the two groups; B: Comparison of serum levels of IL-8 between the two groups; C: Comparison of serum levels of hs-CRP between the two groups; Note: Compared with control group after five days of intervention, *P<0.05. Compared with control group before intervention, ###P<0.001. Compared with observation group before intervention, +++P<0.001. Note: IL-8: Interleukin-8; hs-CRP: hs-C-reactive protein.
Comparison of adverse reactions between the two groups
There was no significant difference in the incidence of nausea, vomiting, rash, abdominal pain and diarrhea between the two groups after intervention (all P>0.05, Table 3).
Table 3.
Comparison of adverse reactions between the two groups
| Adverse reactions | Observation (n=105) | Control (n=105) | χ 2 | P |
|---|---|---|---|---|
| Nausea | 17 (16.19%) | 10 (9.52%) | 7.253 | 0.743 |
| Vomit | 21 (20.00%) | 32 (30.48%) | 6.378 | 0.158 |
| Rash | 20 (19.05%) | 31 (29.52%) | 3.721 | 0.842 |
| Abdominal pain | 34 (32.38%) | 33 (31.43%) | 4.161 | 0.151 |
| Diarrhea | 15 (14.29%) | 7 (6.67%) | 9.837 | 0.482 |
| Total incidence | 38 (36.19%) | 46 (43.81%) | 6.693 | 0.232 |
Comparison of duration of clinical symptoms and hospitalization time between the two groups
The durations of cough, fever, pulmonary rales, lung shadow, and hospitalization time in the observation group were significantly shorter than those in the control group (all P<0.05), as shown in Table 4.
Table 4.
Comparison of duration of clinical symptoms and hospitalization time between the two groups
| Clinical symptoms and hospitalization time | Observation (n=105) | Control (n=105) | t | P |
|---|---|---|---|---|
| The duration of cough | 6.20±0.90 | 8.02±0.95 | 1.702 | 0.041 |
| The duration of fever | 5.48±0.84 | 7.93±0.94 | 2.101 | 0.031 |
| The duration of pulmonary rales | 6.02±1.25 | 7.67±1.52 | 2.032 | 0.026 |
| The duration of lung shadow | 8.87±2.31 | 11.68±2.48 | 4.161 | 0.021 |
| Hospitalization time | 7.24±3.22 | 12.97±4.47 | 6.837 | 0.012 |
Discussion
Immunocompromise is closely associated with the development of severe pneumonia, and enhancing immune function is a treatment for pneumonia that has been increasingly recognized in recent studies [17-19]. Human immunoglobulins are antibodies extracted from plasma that play an important role in the primary immune response [20-23]. In addition, immunoglobulins can impede apoptosis of immune cells and enhance immune function [24,25]. In our study, human immunoglobulin was additionally added to conventional antibiotics for the treatment of pediatric severe pneumonia, and the overall effective rate of children in the observation group was higher than that of the control group. The cough relief time and temperature recovery time were shorter in the observation group than those in the control group. These results suggest that human immunoglobulin can improve the efficacy and clinical symptoms in the treatment of pediatric severe pneumonia.
This study further analyzed the changes in immune biomarkers before and after the use of human immunoglobulins. IgA, IgG and IgM are immunoglobulins involved in humoral immune responses and are able to recognize and bind to pathogens and kill them through immune responses. When immunoglobulins are not sufficient, pathogens cannot be destroyed in time after their invasion. During persistent pathogen infection, the secretion of immunoglobulins is further suppressed, which is detrimental to pathogen clearance [26,27]. Analysis of immunoglobulins showed that serum IgA, IgG and IgM levels increased significantly in both groups after treatment. The trend of increasing immunoglobulins after treatment was more pronounced in the observation group than that in the control group. This indicates that human immunoglobulin treatment for pediatric severe pneumonia can increase immunoglobulin concentrations and improve the humoral immune system.
The disruption of Th1/Th2 balance in the body is an important mechanism of decreased immunoglobulin secretion caused by pathogenic infections [28,29]. The shift of Th1 to Th2 in mycoplasma pneumonia favors a compensatory enhancement of humoral immunity, as evidenced by increased secretion of IFN-γ and IL-4, with a more pronounced increase in IL-4 and a decrease in the IFN-γ/IL-4 ratio [30]. The serum levels of IL-4, INF-γ and INF-γ/IL-4 after five days of intervention were obviously lower than those before intervention in the observation group. This study suggests that the use of human immunoglobulin in the treatment of pediatric severe pneumonia can regulate the Th1/Th2 balance and avoid excessive activation of Th2.
Persistent mycoplasma infection during mycoplasma pneumonia can activate the inflammatory response in vivo. The activation of the inflammatory response in children with severe pneumonia is intense, and various inflammatory mediators are released in a cascade. hs-CRP is an acute temporal protein synthesized by hepatocytes, and pro-inflammatory factors can stimulate hepatocytes to secrete large amounts of hs-CRP during the activation of inflammatory response [31-37]. The results of this study showed that the levels of IL-6, IL-8 and hsCRP in the peripheral blood of both groups were significantly lower than those before treatment, and the levels in the observation group were significantly lower than those in the control group. This result suggests that human immunoglobulin can significantly suppress the inflammatory response in pediatric severe pneumonia.
Although our study illustrates the effectiveness of immunoglobulins in the treatment of severe mycoplasma pneumonia in children, there are still some limitations. This is a retrospective single-center study with small sample size and only focused on mycoplasma pneumonia. In future studies, we will conduct a number of prospective studies and expand the types of disease.
In conclusion, human immunoglobulin combined with antibiotics for the treatment of pediatric severe pneumonia can improve the efficacy, shorten the duration of clinical symptoms, improve immune function, and suppress the inflammatory response.
Acknowledgements
This work was supported by Hebei Medical Science Research Program (No. 20220331).
Disclosure of conflict of interest
None.
References
- 1.de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. 2020;396:786–798. doi: 10.1016/S0140-6736(20)31550-6. [DOI] [PubMed] [Google Scholar]
- 2.Mizgerd JP. Pathogenesis of severe pneumonia: advances and knowledge gaps. Curr Opin Pulm Med. 2017;23:193–197. doi: 10.1097/MCP.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitnun A, Ford-Jones EL, Petric M, MacGregor D, Heurter H, Nelson S, Johnson G, Richardson S. Acute childhood encephalitis and mycoplasma pneumoniae. Clin Infect Dis. 2001;32:1674–1684. doi: 10.1086/320748. [DOI] [PubMed] [Google Scholar]
- 4.Eslamy HK, Newman B. Pneumonia in normal and immunocompromised children: an overview and update. Radiol Clin North Am. 2011;49:895–920. doi: 10.1016/j.rcl.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izumikawa K. Clinical features of severe or fatal mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:800. doi: 10.3389/fmicb.2016.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J, Liu M, Ye Z, Tan T, Liu X, You X, Zeng Y, Wu Y. Insights into the pathogenesis of mycoplasma pneumoniae (Review) Mol Med Rep. 2016;14:4030–4036. doi: 10.3892/mmr.2016.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of mycoplasma pneumoniae: an update. Indian J Med Microbiol. 2016;34:7–16. doi: 10.4103/0255-0857.174112. [DOI] [PubMed] [Google Scholar]
- 8.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daxboeck F, Blacky A, Seidl R, Krause R, Assadian O. Diagnosis, treatment, and prognosis of mycoplasma pneumoniae childhood encephalitis: systematic review of 58 cases. J Child Neurol. 2004;19:865–71. doi: 10.1177/08830738040190110401. [DOI] [PubMed] [Google Scholar]
- 10.Lee KL, Lee CM, Yang TL, Yen TY, Chang LY, Chen JM, Lee PI, Huang LM, Lu CY. Severe mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010-2019. J Formos Med Assoc. 2021;120:281–291. doi: 10.1016/j.jfma.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Meyer Sauteur PM, Streuli JC, Iff T, Goetschel P. Mycoplasma pneumoniae-associated encephalitis in childhood--nervous system disorder during or after a respiratory tract infection. Klin Padiatr. 2011;223:209–213. doi: 10.1055/s-0031-1271717. [DOI] [PubMed] [Google Scholar]
- 12.Watkins LKF, Olson D, Diaz MH, Lin X, Demirjian A, Benitez AJ, Winchell JM, Robinson CC, Bol KA, Glodé MP, Dominguez SR, Miller LA, Kutty PK. Epidemiology and molecular characteristics of mycoplasma pneumoniae during an outbreak of M. pneumoniae-associated Stevens-Johnson syndrome. Pediatr Infect Dis J. 2017;36:564–571. doi: 10.1097/INF.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruzeau C, Cook-Moreau J, Pinaud E, Le Noir S. Contribution of immunoglobulin enhancers to B cell nuclear organization. Front Immunol. 2022;13:877930. doi: 10.3389/fimmu.2022.877930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barragan-Galvez JC, Gonzalez-Orozco M, Hernandez-Flores A, Maravillas-Montero JL, Chavez-Guerrero Y, Ortiz-Navarrete V. Prokaryotic expression of the immunoglobulin’s domains of CRTAM to characterize a monoclonal antibody. Protein J. 2020;39:224–231. doi: 10.1007/s10930-020-09896-y. [DOI] [PubMed] [Google Scholar]
- 15.Respiratory Group of Pediatric Branch of Chinese Medical Association. Expert consensus on diagnosis and treatment of mycoplasma pneumoniae pneumonia in children (2015 Edition) J Chin Appl Clin Peadiatrics. 2015;30:1304–1308. [Google Scholar]
- 16.Krafft C, Christy C. Mycoplasma pneumonia in children and adolescents. Pediatr Rev. 2020;41:12–19. doi: 10.1542/pir.2018-0016. [DOI] [PubMed] [Google Scholar]
- 17.Mansel JK, Rosenow EC 3rd, Smith TF, Martin JW Jr. Mycoplasma pneumoniae pneumonia. Chest. 1989;95:639–646. doi: 10.1378/chest.95.3.639. [DOI] [PubMed] [Google Scholar]
- 18.Smith LG. Mycoplasma pneumonia and its complications. Infect Dis Clin North Am. 2010;24:57–60. doi: 10.1016/j.idc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. 2017;30:747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen PS, Halber MD, Putman CE. Mycoplasma pneumonia. CRC Crit Rev Diagn Imaging. 1980;12:385–415. [PubMed] [Google Scholar]
- 22.Narita M. Pathogenesis of extrapulmonary manifestations of mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16:162–169. doi: 10.1007/s10156-010-0044-x. [DOI] [PubMed] [Google Scholar]
- 23.Intravenous immunoglobulin (IVIG) Med Lett Drugs Ther. 2006;48:101–103. [PubMed] [Google Scholar]
- 24.Rodriguez MM, Wagner-Weiner L. Intravenous immunoglobulin in pediatric rheumatology: when to use it and what is the evidence. Pediatr Ann. 2017;46:e19–e24. doi: 10.3928/19382359-20161214-01. [DOI] [PubMed] [Google Scholar]
- 25.Watad A, Amital H, Shoenfeld Y. Intravenous immunoglobulin: a biological corticosteroid-sparing agent in some autoimmune conditions. Lupus. 2017;26:1015–1022. doi: 10.1177/0961203317696589. [DOI] [PubMed] [Google Scholar]
- 26.Gonçalves R, Gata L, Brett A. Mycoplasma pneumoniae-associated mucositis. BMJ Case Rep. 2021;14:e239086. doi: 10.1136/bcr-2020-239086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai TA, Tsai CK, Kuo KC, Yu HR. Rational stepwise approach for mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2021;54:557–565. doi: 10.1016/j.jmii.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Butcher MJ, Zhu J. Recent advances in understanding the Th1/Th2 effector choice. Fac Rev. 2021;10:30. doi: 10.12703/r/10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15–44. doi: 10.1007/978-94-017-9487-9_2. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Cui J, Cui L, Luo Q, Cao Q, Yuan W, Zhang H. The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem Cell Res Ther. 2019;10:214. doi: 10.1186/s13287-019-1313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcoba G, Keitel K, Maspoli V, Lacroix L, Manzano S, Gehri M, Tabin R, Gervaix A, Galetto-Lacour A. A three-step diagnosis of pediatric pneumonia at the emergency department using clinical predictors, C-reactive protein, and pneumococcal PCR. Eur J Pediatr. 2017;176:815–824. doi: 10.1007/s00431-017-2913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karakioulaki M, Stolz D. Biomarkers in pneumonia-beyond procalcitonin. Int J Mol Sci. 2019;20:2004. doi: 10.3390/ijms20082004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Florin TA, Ambroggio L, Brokamp C, Zhang Y, Rattan M, Crotty E, Belsky MA, Krueger S, Epperson TN 4th, Kachelmeyer A, Ruddy R, Shah SS. Biomarkers and disease severity in children with community-acquired pneumonia. Pediatrics. 2020;145:e20193728. doi: 10.1542/peds.2019-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang C, Mao Y, Jiang M, Yin W. Serum sTREM-1 and CXCL-16 levels in children with mycoplasma pneumoniae pneumonia and their diagnostic value. Evid Based Complement Alternat Med. 2021;2021:7179796. doi: 10.1155/2021/7179796. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Wang J, Zhao Y, Pan L, He X, Zhang X. The relationship between the expression of serum IL-18 mRNA, CC16, and sTREM-1 and the severity and prognosis of ventilator-associated pneumonia in elderly patients. Ann Palliat Med. 2021;10:12767–12774. doi: 10.21037/apm-21-3511. [DOI] [PubMed] [Google Scholar]
- 36.Velásquez S, Matute JD, Gámez LY, Enríquez LE, Gómez ID, Toro F, Valencia ML, De La Rosa G, Patiño PJ, Jaimes FA. Characterization of nCD64 expression in neutrophils and levels of s-TREM-1 and HMGB-1 in patients with suspected infection admitted in an emergency department. Biomedica. 2013;33:643–652. doi: 10.7705/biomedica.v33i4.805. [DOI] [PubMed] [Google Scholar]
- 37.van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–445. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]


