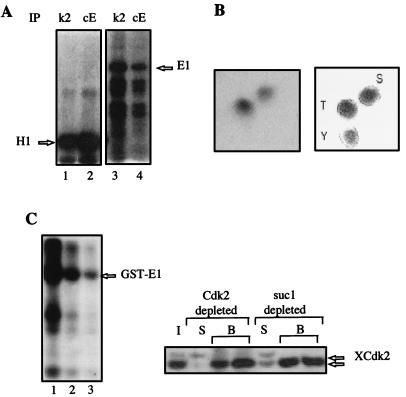
FIG. 4.
The viral helicase E1 is a substrate of cyclin E-Cdk2. (A) E1 is phosphorylated by cyclin E-Cdk2 in vitro. Xenopus HSS were immunoprecipitated (IP) with cyclin E antiserum (cE) or Cdk2 antiserum (k2), and the associated protein kinase activity was tested by using histone H1 (lanes 1 and 2) or E1 (lanes 3 and 4) as substrate in the presence of [γ-32P]ATP. Phosphorylated proteins were resolved by SDS-PAGE and visualized by autoradiography. (B) Cyclin E-Cdk2 phosphorylates E1 on serine and threonine. Purified E1 protein was phosphorylated by Cdk2-associated kinase as in panel A, and radiolabeled E1 protein was hydrolyzed to amino acids that were separated by two-dimensional electrophoresis on thin-layer plates (left); the mobilities of phosphoserine (S), phosphothreonine (T), and phosphotyrosine (Y) standards are shown on the right. (C) E1 is a substrate of cyclin E-Cdk2 in Xenopus egg extracts. GST-E1 beads were incubated in undepleted Xenopus egg extract (lane 1), in suc1-depleted extract (lane 2), or in Cdk2-depleted extract (lane 3) in the presence of [γ-32P]ATP. Xenopus Cdk2 (XCdk2) depletion was monitored by Western blotting (right). I, input extract (half amount); B, beads; S, supernatant after incubation with beads.