Fig. 6.
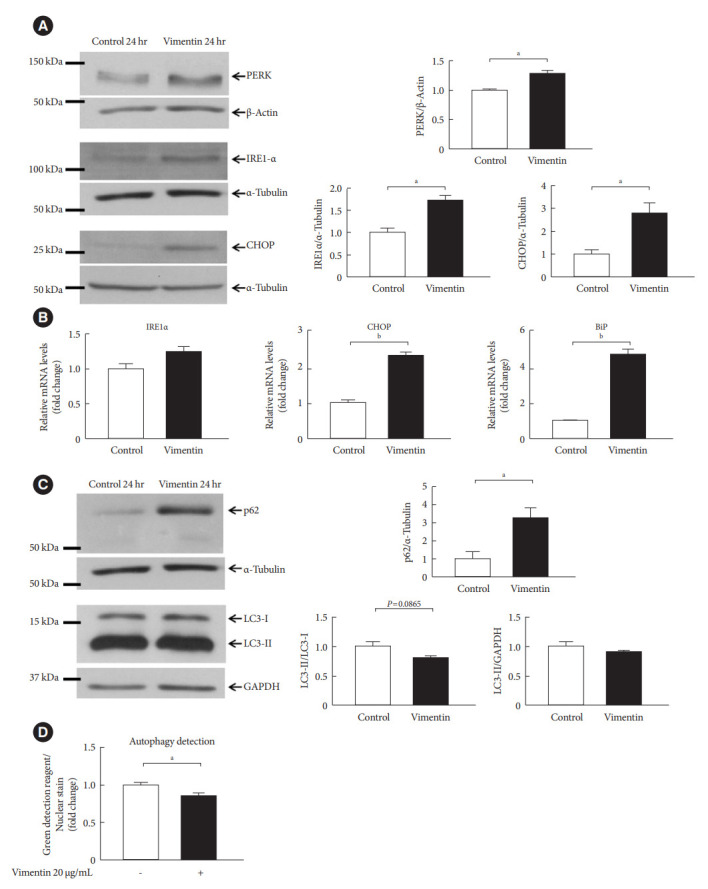
Extracellular vimentin induces endoplasmic reticulum (ER) stress and impairs autophagy. (A) Western blot analyses for protein kinase R-like ER kinase (PERK), inositol-requiring enzyme 1 α (IRE1α), and C/EBP homologous protein (CHOP) were performed using lysates of 3T3-L1-derived adipocytes treated with or without recombinant vimentin (20 µg/mL) for 24 hours. Band quantification was performed using β-actin and α-tubulin as internal controls (n=3). (B) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analyses for IRE1α, CHOP, and binding immunoglobulin protein (BiP) were performed using RNA from 3T3-L1-derived adipocytes treated with or without vimentin (20 µg/mL) for 24 hours. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control (n=4). (C) Western blot analysis for p62, light chain 3 (LC3)-I, and LC3-II were performed using lysates of 3T3-L1-derived adipocytes treated with or without recombinant vimentin (20 µg/mL) for 24 hours. Band quantification was performed using α-tubulin and GAPDH as internal controls (n=3). (D) Autophagic vacuoles in 3T3-L1-derived adipocytes treated with or without recombinant vimentin (20 µg/mL) for 24 hours, were measured. Data were normalized to Hoechst 33342 staining in the adipocytes used in the assay (n=5). aP<0.05, bP<0.001.
