Since the seminal 2014 publication describing two distinct phenotypes, hyper- and hypoinflammatory, in acute respiratory distress syndrome (ARDS) (1), the field has made major progress in characterizing critical illness heterogeneity. Host–response phenotyping has shown impressive replicability in several independent cohorts of patients with ARDS, hypoxemic respiratory failure without ARDS (2, 3), sepsis, and acute pancreatitis (4). With unsupervised classification approaches leveraging both clinical variables and, most frequently, plasma protein biomarker concentrations, patients with the same syndromic diagnosis could be classified into distinct phenotypes, characterized by marked differences in organ dysfunction indices and clinical outcomes. Retrospective analyses of randomized clinical trials suggest that phenotypes also partially explain heterogeneity of treatment effect. This suggests potential utility for predictive enrichment in clinical trials, an approach that awaits prospective validation (4).
Despite these advances, the biological underpinnings of the observed phenotypes remain enigmatic. For example, probabilistic classification to a hyper- versus hypoinflammatory phenotype by a regression model does not help us understand the biology of interindividual differences in biomarker profiles and clinical outcomes for patients with the same diagnosis (e.g., ARDS). Admittedly, phenotyping research has been informed by an implicit hypothesis in most studies to date: phenotypes must represent interindividual variation of host responses to similar insults, or alternatively, the host matters more than the insult in phenotypic classification.
Ten years later in this issue of the Journal, Neyton and colleagues (pp. 805–815) offer a potential leap forward in our conceptual understanding of sepsis phenotyping, by studying both the critically ill host and the illness-inducing pathogens (5). The authors analyzed host blood transcriptomics and plasma microbial DNA metagenomics in 189 patients with sepsis in the emergency department, with 40% classified with the hyperinflammatory phenotype. All patients were treated with mechanical ventilation and/or vasopressors, representing the most severely ill subset of the observational EARLI (Early Assessment of Renal and Lung Injury) study, which prompted the collection of a PAXgene (BD Biosciences) tube for sequencing.
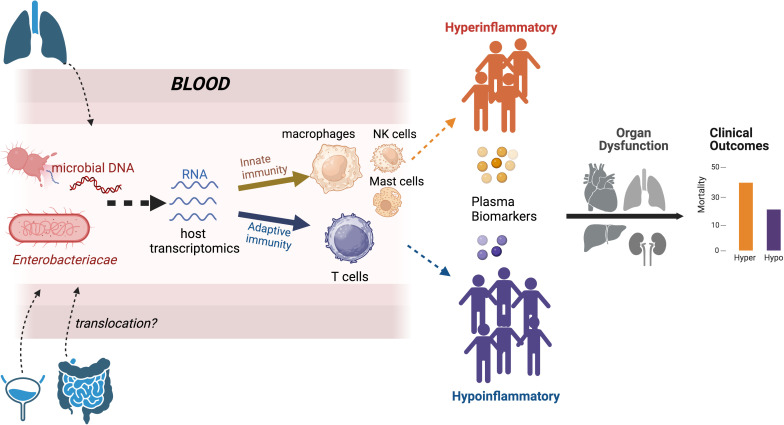
On the host side, bulk RNA transcriptomics unveiled the expected complexity of transcriptional programs underlying phenotype classifications. Hyperinflammatory patients had increased expression of genes and pathways related to innate immune response, including the PD-1/PD-L1 pathway and IL-8 signaling, whereas hypoinflammatory patients exhibited increased expression of genes related to adaptive immunity and T-cell responses. In silico analysis of inferred cell type differentials also supported the notion of an innate immunity “hyperinflammatory” phenotype, with increased proportions of macrophages and mast and natural killer cells, versus an adaptive immunity “hypoinflammatory” phenotype with greater abundance of CD4 T cells. Transcriptional analyses also revealed differences in metabolic pathways, with enrichment of oxidative phosphorylation, glycolysis, and cholesterol biosynthesis pathways in hyperinflammatory patients. This transcriptional activation aligns with previous research indicating metabolic dysfunction in hyperinflammatory patients, marked by elevated lactate concentrations, mitochondrial dysfunction, and dysregulated lipid metabolism (6, 7). Nonetheless, the transcriptomic analyses must be considered within the context of marked differences in bacteremia, with 49% versus 8% of bacteremia rates between hyperinflammatory versus hypoinflammatory patients. Comparisons with publicly available external datasets showed modest but statistically significant reproducibility of transcriptional signatures by phenotype, especially those defined by plasma biomarkers.
On the microbial side, plasma metagenomic DNA sequencing revealed further insights. The plasma of hyperinflammatory patients had higher microbial DNA biomass, with higher abundance of bacterial sequences belonging to a single bacterial species (dominance), perhaps related to the circulating DNA of the causal pathogen. These findings parallel prior observations of a discriminatory signature with “molecularly nonsterile” blood. Hyperinflammatory patients with pneumonia or acute respiratory failure had higher concentrations of plasma microbial cell–free DNA (8) and circulating β-d-glucan (9) and impaired alternative complement pathway function associated with higher risk of bloodstream infections (10). In the context of coronavirus disease (COVID-19) pneumonia, plasma concentrations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA (RNA-emia), as well as bacterial cell–free DNA from probable superinfecting pathogens, were correlated with several host plasma cytokines (11, 12), further supporting the concept of circulating pathogen load contributing to the inflammatory response.
The biological impact of such “nonsterile” hyperinflammatory blood remains unclear, but Neyton and colleagues’ (5) novel findings have both diagnostic and mechanistic promise. Circulating microbial fragments in the blood may offer a noninvasive and sensitive option for etiologic pathogen diagnosis in sepsis. Such microbial fragments may also act as pathogen-associated molecular patterns (PAMPs) that activate pattern recognition receptors (13), resulting in the downstream biomarker profiles we use to phenotype patients. Microbes and their PAMPs may be causally related to the clinical presentation (e.g., sepsis), and they may proximally determine immune pathways that are ultimately summarized as phenotypes (Figure 1). If there are mechanistic links between microbial PAMPs and host phenotypes, then interactions between PAMPs and pattern recognition receptors may offer proximal opportunities for therapeutic targeting.
Figure 1.
Conceptual advancement in understanding host–response phenotypes in critical illness. Current classification approaches by plasma biomarker concentrations offer robust associations with organ dysfunction and the prediction of clinical outcomes. The study by Neyton and colleagues (5) provides insights into more proximal determinants of phenotypes. Blood host transcriptomics analyses showed increased expression of genes and pathways involved in innate immune cells in hyperinflammatory patients, whereas hypoinflammatory patients had increased expression of genes involved in T-cell responses. Microbial DNA metagenomics showed a higher amount of microbial DNA in hyperinflammatory patients, who were also enriched for DNA belonging to the Enterobacteriaceae family. Enterobacteriaceae are typical enteric-origin taxa, raising the hypothesis of possible gut-to-blood translocation in hyperinflammatory patients. Hyper = hyperinflammatory; Hypo = hypoinflammatory; NK = natural killer.
Careful observation of differential abundance of microbial species by plasma metagenomics led the authors to another provocative hypothesis. Escherichia coli was more abundant in hyperinflammatory patients, who also had higher dominance of their bacterial reads signal by Enterobacteriaceae taxa, a prototypical bacterial family of enteric origin. This plasma Enterobacteriaceae signal could not be corroborated by microbiological cultures of the respiratory or urinary tracts. Therefore, Enterobacteriaceae, either in complete living forms or in cellular fragments, may have leaked into the bloodstream from the gut (i.e., gut-to-blood translocation) (14). Gut translocation has been long proposed as a biologically plausible mechanism, although convincingly observing its occurrence or accurately quantifying its components during critical illness has proved to be impractical. The association of plasma concentrations of enteric-origin taxa DNA with the hyperinflammatory phenotype suggests that detailed multicompartment study of microbiota may help disentangle the attractive yet elusive mechanism of translocation.
Despite the novelty and conceptual advancements offered by Neyton and colleagues (5), their results ought to be interpreted as hypothesis generating. The small sample size, selection of the sickest subset of early presenters with sepsis, and single-center design only partially validated by external datasets mandate independent replication in broader patient populations. Longitudinal study of host–microbiota interactions is also needed to help understand how phenotypes may change during immunity phase transitions or with evolving microbiota communities in the ICU. The host–microbiota relationships revealed during the hyperacute phase of sepsis suggest that translation into clinical practice will require advancements in molecular assay capabilities beyond emerging point-of-care protein biomarker analyzers, to rapidly capture immune and microbiota signatures in clinically relevant turnaround times.
Yet Neyton and colleagues’ study (5) marks a conceptual shift from merely discovering prognostic phenotypes in critical illness to an era of informative delabeling of inevitably simplistic phenotypic labels. In this new “era,” by embracing both host and microbial signatures of phenotypically “bad blood,” previously enigmatic phenotypes gain clarity within the context of the timeless “immunity versus pathogen” battle. Integrative host and microbial phenotyping, extending beyond isolated hyper- versus hypoinflammatory classifications, may thus enable more precise targeting of treatable traits, moving us closer to the realization of precision medicine in critical care.
Footnotes
Supported by the University of Pittsburgh Clinical and Translational Science Institute COVID-19 Pilot Award, NHLBI grant R03 HL162655, and the American Lung Association COVID-19 Respiratory Virus Research Award (G.D.K.) and U.S. Department of Veterans Affairs Biomedical Laboratory R&D Service Career Development Award IK2 BX004886 (W.B.).
Originally Published in Press as DOI: 10.1164/rccm.202401-0004ED on February 2, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, NHLBI ARDS Network Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med . 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kitsios GD, Yang L, Manatakis DV, Nouraie M, Evankovich J, Bain W, et al. Host-response subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med . 2019;47:1724–1734. doi: 10.1097/CCM.0000000000004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heijnen NFL, Hagens LA, Smit MR, Cremer OL, Ong DSY, van der Poll T, et al. Biological subphenotypes of acute respiratory distress syndrome show prognostic enrichment in mechanically ventilated patients without acute respiratory distress syndrome. Am J Respir Crit Care Med . 2021;203:1503–1511. doi: 10.1164/rccm.202006-2522OC. [DOI] [PubMed] [Google Scholar]
- 4. Sinha P, Meyer NJ, Calfee CS. Biological phenotyping in sepsis and acute respiratory distress syndrome. Annu Rev Med . 2023;74:457–471. doi: 10.1146/annurev-med-043021-014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neyton LPA, Sinha P, Sarma A, Mick E, Kalantar K, Chen S, et al. Host and microbe blood metagenomics reveals key pathways characterizing critical illness phenotypes. Am J Respir Crit Care Med . 2024;209:805–815. doi: 10.1164/rccm.202308-1328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suber TL, Wendell SG, Mullett SJ, Zuchelkowski B, Bain W, Kitsios GD, et al. Serum metabolomic signatures of fatty acid oxidation defects differentiate host-response subphenotypes of acute respiratory distress syndrome. Respir Res . 2023;24:136. doi: 10.1186/s12931-023-02447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alipanah-Lechner N, Neyton L, Mick E, Willmore A, Leligdowicz A, Contrepois K, et al. Plasma metabolic profiling implicates dysregulated lipid metabolism and glycolytic shift in hyperinflammatory ARDS. Am J Physiol Lung Cell Mol Physiol . 2023;324:L297–L306. doi: 10.1152/ajplung.00278.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang H, Haidar G, Al-Yousif NS, Zia H, Kotok D, Ahmed AA, et al. Circulating microbial cell-free DNA is associated with inflammatory host-responses in severe pneumonia. Thorax . 2021;76:1231–1235. doi: 10.1136/thoraxjnl-2020-216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kitsios GD, Kotok D, Yang H, Finkelman MA, Zhang Y, Britton N, et al. Plasma 1,3-β-d-glucan levels predict adverse clinical outcomes in critical illness. JCI Insight . 2021;6:e141277. doi: 10.1172/jci.insight.141277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bain W, Li H, van der Geest R, Moore SR, Olonisakin TF, Ahn B, et al. Increased alternative complement pathway function and improved survival during critical illness. Am J Respir Crit Care Med . 2020;202:230–240. doi: 10.1164/rccm.201910-2083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobs JL, Bain W, Naqvi A, Staines B, Castanha PMS, Yang H, et al. Severe acute respiratory syndrome coronavirus 2 viremia is associated with coronavirus disease 2019 severity and predicts clinical outcomes. Clin Infect Dis . 2022;74:1525–1533. doi: 10.1093/cid/ciab686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lisius G, Duttagupta R, Ahmed AA, Hensley M, Al-Yousif N, Lu M, et al. Noninvasive diagnosis of secondary infections in COVID-19 by sequencing of plasma microbial cell-free DNA. iScience . 2023;26:108093. doi: 10.1016/j.isci.2023.108093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiersinga WJ, van der Poll T. Immunopathophysiology of human sepsis. EBioMedicine . 2022;86:104363. doi: 10.1016/j.ebiom.2022.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nath S, Kitsios GD, Bos LDJ. Gut-lung crosstalk during critical illness. Curr Opin Crit Care . 2023;29:130–137. doi: 10.1097/MCC.0000000000001015. [DOI] [PubMed] [Google Scholar]