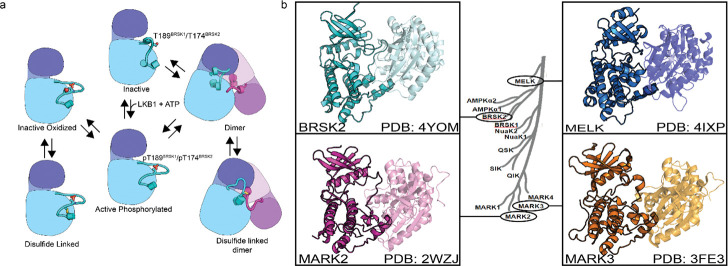
Figure 8:
(a) Model of BRSK1/2 regulation. Schematic diagram demonstrating ways in which residues within BRSK kinases permit fine-tuning of catalytic activity through a variety of oxidative modifications, potentially including inter and intramolecular disulfide bonds. Cartoon representation of kinase domain with N-lobe colored dark blue/purple and the C-lobe colored light blue/purple. (b) ARK family member BRSK2, MELK, and MARK2/3 crystal structures demonstrate the ability to form asymetric dimers bringing T + 2 cys into proximity. Crystal structures for MARK2, and MELK both contain intermolecular disulfide bonds between T + 2 cys (Marx et al. 2010; Marx et al. 2006; Murphy et al. 2007; Cao et al. 2013).